
Contributions
Abstract: EP734
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Prognosis of relapsed/refractory (R/R) B-cell acute lymphoid leukemia (ALL) is poor, particularly in patients relapsing after allogeneic hematopoietic cell transplantation (alloHCT). In this setting, several chimeric antigen receptor products targeting CD19 have been capable of achieving complete response (CR) rates of 70 to 85%. Nevertheless, a significant proportion of patients relapse despite having achieved a minimal residual disease (MRD) negative CR.
Aims
To identify factors associated with progression-free survival (PFS) in patients with R/R B-ALL treated with ARI-0001 (Academic CART19 cells).
Methods
We report the outcome of all patients with R/R B-ALL consecutively treated with ARI-0001 cells in two centers (adult and pediatric) from Jul 2017 to Dec 2020, including patients treated in the CART19-BE-01 trial and the consecutive compassionate use program. All patients signed informed consent and all protocols were approved by the Ethics Committees and the Spanish Medicines Agency. Lymphodepletion was performed with fludarabine (90 mg/m2) and cyclophosphamide (900 mg/m2), followed by the infusion of 0.1-5 x106 ARI-0001 cells/kg in a single or fractionated manner. We analyzed the impact on PFS of the following variables: age (+/- 18 yr and +/- 25 yr), prior treatment lines (+/- 4), prior immunotherapy (blinatumomab and inotuzumab), prior alloHCT, tumor burden (+/- 5% blasts at bone marrow), type of dosage (single vs fractionated dosing), and loss of B-cell aplasia (BCA). The statistical analysis was performed using Kaplan Meier / log rank test for all variables except for B-cell aplasia, which was introduced as a time-dependent covariate in a Cox regression.
Results
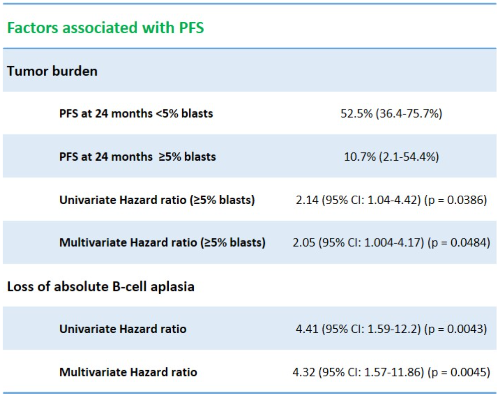
A total of 53 patients with R/R B-ALL received ARI-0001 cells. Median age at infusion was 30 years (3-68), including 10 pediatric patients (19%), and 55% were men. Patients had a median of 4 prior treatment lines (2-8), including blinatumomab 23% (12/53), inotuzumab 53% (28/53) and alloHCT 79% (42/53). All patients received lymphodepletion before receiving ARI-0001 cells. Treatment was fractionated in 72% of patients. The median PFS for the entire series was 13.5 months (95% CI: 7.14-not available), with a median follow-up for survivors of 19 months (range: 2-40 months). PFS at 12 and 24 months was 50.9% (95% CI: 38.4-67.4%) and 32.9% (95% CI: 20.6-52.6%), respectively. The median overall-survival (OS) for the entire series was 29.2 months (95% CI: 15-not available). OS at 12 and 24 months was 70.2% (95% CI: 58.1-84.8%) and 53.9% (95% CI: 40.5-71.8%), respectively. By univariate analysis, only two variables had an impact on PFS (table 1): tumor burden (+/- 5% of blasts at screening), with a PFS at 24 months of 52.5% (36.4-75.7%) vs. 10.7% (2.1-54.4%) and a hazard ratio (HR) of 2.14 (95% 1.04-4.42) for patients with 5% or more blasts at screening (p = 0.0386). On the other hand, loss of BCA had a HR of 4.41 (95% CI: 1.59-12.2), p = 0.0043. Both variables (tumor burden and loss of BCA) were also confirmed in the multivariate model, with a HR of 2.05 (1.004-4.17) for patients with 5% or more blasts at screening (p = 0.0484) and a HR of 4.32 (1.57-11.86) for patients with loss of BCA (p = 0.0045).
Conclusion
ARI-0001 therapy was able to achieve long term remissions in this population of R/R B-ALL patients across all patient subgroups. Nevertheless, patients referred for ARI-0001 cell therapy with 5% or more of bone marrow blasts and those who lost B-cell aplasia after ARI-0001 cell therapy had a shorter PFS.
Keyword(s): ALL, CAR-T, CD19, Prognostic factor
Abstract: EP734
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Prognosis of relapsed/refractory (R/R) B-cell acute lymphoid leukemia (ALL) is poor, particularly in patients relapsing after allogeneic hematopoietic cell transplantation (alloHCT). In this setting, several chimeric antigen receptor products targeting CD19 have been capable of achieving complete response (CR) rates of 70 to 85%. Nevertheless, a significant proportion of patients relapse despite having achieved a minimal residual disease (MRD) negative CR.
Aims
To identify factors associated with progression-free survival (PFS) in patients with R/R B-ALL treated with ARI-0001 (Academic CART19 cells).
Methods
We report the outcome of all patients with R/R B-ALL consecutively treated with ARI-0001 cells in two centers (adult and pediatric) from Jul 2017 to Dec 2020, including patients treated in the CART19-BE-01 trial and the consecutive compassionate use program. All patients signed informed consent and all protocols were approved by the Ethics Committees and the Spanish Medicines Agency. Lymphodepletion was performed with fludarabine (90 mg/m2) and cyclophosphamide (900 mg/m2), followed by the infusion of 0.1-5 x106 ARI-0001 cells/kg in a single or fractionated manner. We analyzed the impact on PFS of the following variables: age (+/- 18 yr and +/- 25 yr), prior treatment lines (+/- 4), prior immunotherapy (blinatumomab and inotuzumab), prior alloHCT, tumor burden (+/- 5% blasts at bone marrow), type of dosage (single vs fractionated dosing), and loss of B-cell aplasia (BCA). The statistical analysis was performed using Kaplan Meier / log rank test for all variables except for B-cell aplasia, which was introduced as a time-dependent covariate in a Cox regression.
Results
A total of 53 patients with R/R B-ALL received ARI-0001 cells. Median age at infusion was 30 years (3-68), including 10 pediatric patients (19%), and 55% were men. Patients had a median of 4 prior treatment lines (2-8), including blinatumomab 23% (12/53), inotuzumab 53% (28/53) and alloHCT 79% (42/53). All patients received lymphodepletion before receiving ARI-0001 cells. Treatment was fractionated in 72% of patients. The median PFS for the entire series was 13.5 months (95% CI: 7.14-not available), with a median follow-up for survivors of 19 months (range: 2-40 months). PFS at 12 and 24 months was 50.9% (95% CI: 38.4-67.4%) and 32.9% (95% CI: 20.6-52.6%), respectively. The median overall-survival (OS) for the entire series was 29.2 months (95% CI: 15-not available). OS at 12 and 24 months was 70.2% (95% CI: 58.1-84.8%) and 53.9% (95% CI: 40.5-71.8%), respectively. By univariate analysis, only two variables had an impact on PFS (table 1): tumor burden (+/- 5% of blasts at screening), with a PFS at 24 months of 52.5% (36.4-75.7%) vs. 10.7% (2.1-54.4%) and a hazard ratio (HR) of 2.14 (95% 1.04-4.42) for patients with 5% or more blasts at screening (p = 0.0386). On the other hand, loss of BCA had a HR of 4.41 (95% CI: 1.59-12.2), p = 0.0043. Both variables (tumor burden and loss of BCA) were also confirmed in the multivariate model, with a HR of 2.05 (1.004-4.17) for patients with 5% or more blasts at screening (p = 0.0484) and a HR of 4.32 (1.57-11.86) for patients with loss of BCA (p = 0.0045).

Conclusion
ARI-0001 therapy was able to achieve long term remissions in this population of R/R B-ALL patients across all patient subgroups. Nevertheless, patients referred for ARI-0001 cell therapy with 5% or more of bone marrow blasts and those who lost B-cell aplasia after ARI-0001 cell therapy had a shorter PFS.
Keyword(s): ALL, CAR-T, CD19, Prognostic factor


