
Contributions
Abstract: EP730
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Etranacogene dezaparvovec is an investigational gene therapy for hemophilia B (HB) comprising an adeno-associated virus serotype 5 (AAV5) vector containing a codon-optimized Padua variant human factor IX (FIX) transgene with a liver specific promoter. Preliminary data suggest that anti-AAV5 neutralizing antibodies (NAbs) may not preclude successful transduction with etranacogene dezaparvovec.
Aims
A Phase 3 trial (HOPE-B; NCT03569891) was established to assess efficacy and safety of a single dose of etranacogene dezaparvovec (2x1013gc/kg). Adult males with HB (FIX≤2%) receiving routine FIX prophylaxis, with/without AAV5 NAbs, were enrolled into this open-label, single-dose, single-arm trial, following a ≥6 month lead-in. Co-primary endpoints; change in FIX activity (measured by one stage assay) at 26 and 52wks and 52wk annualized bleeding rate compared to lead-in.
Methods
Outcomes at 26wks are analyzed using descriptive statistics and a correlation analysis.
Results
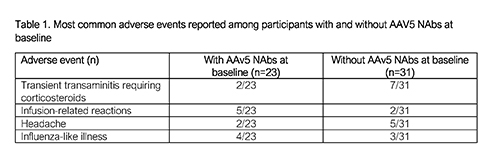
54 participants were dosed and completed 26wks of follow-up, 23 (42.6%) of whom had AAV5 Nabs at baseline (BL) with a median titer of 56.9 (max titer 3212; 1st-3rd quartile 23.3-282.5) and a distribution representative of the general population. One participant (titer 198) received a partial dose and was excluded from efficacy assessments. One participant (titer 3212) did not respond and remained on prophylaxis. All other participants (n=52) discontinued prophylaxis. No correlation of pre-existing NAbs with FIX activity was observed up to a titer of 678 (n=52, r=-0.28 [95% CI -0.51, 0.00], R2=0.078). Mean FIX activity at 26 weeks was 32.7 IU/dl (min <2, max 90.4, 1st-3rd quartile 16.3-42.6, n=22) in participants with NAbs versus 41.3 IU/dl (min 8.4, max 97.1, 1st-3rd quartile 31.3-52.7, n=31) in those without.
No deaths and no inhibitors to FIX were reported.
Conclusion
This study demonstrates successful treatment of participants with generally prevalent titers of pre-existing NAbs to AAV5 with etranacogene dezaparvovec, supporting broad eligibility for AAV5-based therapies.
Keyword(s): AAV, Clinical outcome, Gene therapy, Hemophilia
Abstract: EP730
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Etranacogene dezaparvovec is an investigational gene therapy for hemophilia B (HB) comprising an adeno-associated virus serotype 5 (AAV5) vector containing a codon-optimized Padua variant human factor IX (FIX) transgene with a liver specific promoter. Preliminary data suggest that anti-AAV5 neutralizing antibodies (NAbs) may not preclude successful transduction with etranacogene dezaparvovec.
Aims
A Phase 3 trial (HOPE-B; NCT03569891) was established to assess efficacy and safety of a single dose of etranacogene dezaparvovec (2x1013gc/kg). Adult males with HB (FIX≤2%) receiving routine FIX prophylaxis, with/without AAV5 NAbs, were enrolled into this open-label, single-dose, single-arm trial, following a ≥6 month lead-in. Co-primary endpoints; change in FIX activity (measured by one stage assay) at 26 and 52wks and 52wk annualized bleeding rate compared to lead-in.
Methods
Outcomes at 26wks are analyzed using descriptive statistics and a correlation analysis.
Results
54 participants were dosed and completed 26wks of follow-up, 23 (42.6%) of whom had AAV5 Nabs at baseline (BL) with a median titer of 56.9 (max titer 3212; 1st-3rd quartile 23.3-282.5) and a distribution representative of the general population. One participant (titer 198) received a partial dose and was excluded from efficacy assessments. One participant (titer 3212) did not respond and remained on prophylaxis. All other participants (n=52) discontinued prophylaxis. No correlation of pre-existing NAbs with FIX activity was observed up to a titer of 678 (n=52, r=-0.28 [95% CI -0.51, 0.00], R2=0.078). Mean FIX activity at 26 weeks was 32.7 IU/dl (min <2, max 90.4, 1st-3rd quartile 16.3-42.6, n=22) in participants with NAbs versus 41.3 IU/dl (min 8.4, max 97.1, 1st-3rd quartile 31.3-52.7, n=31) in those without.
No deaths and no inhibitors to FIX were reported.

Conclusion
This study demonstrates successful treatment of participants with generally prevalent titers of pre-existing NAbs to AAV5 with etranacogene dezaparvovec, supporting broad eligibility for AAV5-based therapies.
Keyword(s): AAV, Clinical outcome, Gene therapy, Hemophilia


