
Contributions
Abstract: EP726
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Biology & Translational Research
Background
CD19-targeted chimeric antigen receptor (CAR)-T cell is an approved cell therapy for the treatment of patients diagnosed with several subtypes of B cell lymphoma. Besides, monitoring CAR-T cell levels during follow-up is mandatory in order to ensure treatment success. Multiparametric flow cytometry (MFC) has proven to be an efficient method. However, improving follow-up with complementary approaches, such as qPCR could also be useful. CAR structure of both Tisagenlecleucel (Tisa-cel) (Kymriah®) and Axicabtagén Ciloleucel (Axi-cel) (Yescarta®) shares the FMC63 single chain variable fragment domain, which sequence is known, enabling their monitoring by qPCR.
Aims
To evaluate the utility of FMC63-specific qPCR for the detection of CAR-T cell levels during follow-up in patients treated with this therapy.
Methods
Twenty-nine patients diagnosed with a B-cell lymphoma who underwent CAR-T therapy with Axi-cel (8) and Tisa-cel (21) between June 2019 and January 2021 were included in this study. Peripheral blood (PB) samples were collected in EDTA tubes at days +7, +14, +30 and +90 (23, 24, 25 and 14 samples, respectively) from CAR-T administration, analyzing a total of 86 PB samples. In addition, nine tumor samples from eight patients were obtained from core needle biopsies. DNA was purified from PB samples using the Maxwell 16 Blood DNA Purification Kit (Promega, USA). Tumor samples were cut and disaggregated and DNA was purified using the QIAamp DNA Mini Kit (Qiagen). FMC63-specific qPCR was carried out in DNA samples, using primers and conditions previously described. For CAR-T quantification we first obtained the percentage of CAR-T cells through the ΔΔCt method (using a 100% CAR-T cell DNA sample) followed by a estimation of the absolute number of CAR-T cells from total cell count. MFC was performed on a DxFLEX cytometer (Beckman Coulter), using CD19 (20-291) protein-FITC (ACRO Biosystems). Quantitative variables were expressed as median and range. Comparison between CAR-T cells detection by MFC and qPCR was analyzed using the Pearson correlation test.
Results
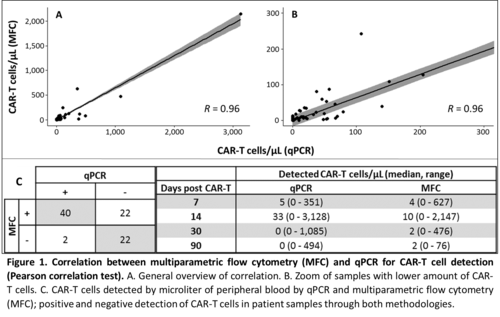
There was an optimal positive correlation between qPCR and MFC for the detection of CAR-T cells, demonstrating the usefulness of qPCR (Figure 1). Median number of CAR-T cells detected by qPCR was greater than those detected by MFC at days +7 and +14, as opposed to days +30 and +90 (Figure 1). This is probably because CAR-T cell contains a variable copy number of FMC63 sequence and at the time of CAR-T expansion (days +7 and +14), these differences could be greater. At later stages, CAR-T cell percentage over the total number of cells is lower and the sensitivity of qPCR method is not comparable to MFC, which is able to analyze a greater number of cells. These facts may also explain the 22 samples in which CAR-T cells were detected by CMF and not by qPCR, since percentage of CAR-T in these samples was lower than 0.5% of total cells. (Figure 1). In respect of tumor samples, CAR-T cells were detected by qPCR in three out nine. Since fresh sample is not needed, qPCR demonstrates its superiority over MFC in terms of detection of CAR-T in tissue samples.
Conclusion
Although qPCR is a reliable method for measuring CAR-T cell levels in patients undergoing this therapy, showing a good correlation with MFC, its sensitivity may not be sufficient at later stages of treatment. Approaches such as the application of qPCR in isolated T-cells or the use of digital PCR, could improve its usefulness in CAR-T cell monitoring.
Keyword(s): B cell lymphoma, CAR-T, Quantitative RT-PCR
Abstract: EP726
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Biology & Translational Research
Background
CD19-targeted chimeric antigen receptor (CAR)-T cell is an approved cell therapy for the treatment of patients diagnosed with several subtypes of B cell lymphoma. Besides, monitoring CAR-T cell levels during follow-up is mandatory in order to ensure treatment success. Multiparametric flow cytometry (MFC) has proven to be an efficient method. However, improving follow-up with complementary approaches, such as qPCR could also be useful. CAR structure of both Tisagenlecleucel (Tisa-cel) (Kymriah®) and Axicabtagén Ciloleucel (Axi-cel) (Yescarta®) shares the FMC63 single chain variable fragment domain, which sequence is known, enabling their monitoring by qPCR.
Aims
To evaluate the utility of FMC63-specific qPCR for the detection of CAR-T cell levels during follow-up in patients treated with this therapy.
Methods
Twenty-nine patients diagnosed with a B-cell lymphoma who underwent CAR-T therapy with Axi-cel (8) and Tisa-cel (21) between June 2019 and January 2021 were included in this study. Peripheral blood (PB) samples were collected in EDTA tubes at days +7, +14, +30 and +90 (23, 24, 25 and 14 samples, respectively) from CAR-T administration, analyzing a total of 86 PB samples. In addition, nine tumor samples from eight patients were obtained from core needle biopsies. DNA was purified from PB samples using the Maxwell 16 Blood DNA Purification Kit (Promega, USA). Tumor samples were cut and disaggregated and DNA was purified using the QIAamp DNA Mini Kit (Qiagen). FMC63-specific qPCR was carried out in DNA samples, using primers and conditions previously described. For CAR-T quantification we first obtained the percentage of CAR-T cells through the ΔΔCt method (using a 100% CAR-T cell DNA sample) followed by a estimation of the absolute number of CAR-T cells from total cell count. MFC was performed on a DxFLEX cytometer (Beckman Coulter), using CD19 (20-291) protein-FITC (ACRO Biosystems). Quantitative variables were expressed as median and range. Comparison between CAR-T cells detection by MFC and qPCR was analyzed using the Pearson correlation test.
Results
There was an optimal positive correlation between qPCR and MFC for the detection of CAR-T cells, demonstrating the usefulness of qPCR (Figure 1). Median number of CAR-T cells detected by qPCR was greater than those detected by MFC at days +7 and +14, as opposed to days +30 and +90 (Figure 1). This is probably because CAR-T cell contains a variable copy number of FMC63 sequence and at the time of CAR-T expansion (days +7 and +14), these differences could be greater. At later stages, CAR-T cell percentage over the total number of cells is lower and the sensitivity of qPCR method is not comparable to MFC, which is able to analyze a greater number of cells. These facts may also explain the 22 samples in which CAR-T cells were detected by CMF and not by qPCR, since percentage of CAR-T in these samples was lower than 0.5% of total cells. (Figure 1). In respect of tumor samples, CAR-T cells were detected by qPCR in three out nine. Since fresh sample is not needed, qPCR demonstrates its superiority over MFC in terms of detection of CAR-T in tissue samples.

Conclusion
Although qPCR is a reliable method for measuring CAR-T cell levels in patients undergoing this therapy, showing a good correlation with MFC, its sensitivity may not be sufficient at later stages of treatment. Approaches such as the application of qPCR in isolated T-cells or the use of digital PCR, could improve its usefulness in CAR-T cell monitoring.
Keyword(s): B cell lymphoma, CAR-T, Quantitative RT-PCR


