
Contributions
Abstract: EP716
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Biology & Translational Research
Background
In spite of high rates of overall response following chimeric antigen receptor (CAR) T cell therapy its curative potential is jeopardized by exhaustion of the CAR T cells conceivably mediated by the immunosuppressive tumor microenvironment and excessive antigen exposure. However, cellular and molecular predictors of clinical response are not fully elucidated.
Aims
To identify phenotypic, transcriptional and cellular mediators of resistance to CAR T immune therapy.
Methods
We performed a broad immunophenotypic and transcriptomic analysis of the T cells from patients (pts) with relapsed/refractory B cell malignancies (n-39: NHL-28, ALL-5), and CLL-6) treated with locally produced CD19 CAR T cells in correlation with pts’ day 28 clinical response. Intact CD3+ T cells that were obtained from peripheral blood (PB) samples pre Flu/Cy lymphodepletion using negative magnetic sorting and the manufactured CAR T products were analyzed. The CD8+ and CD4+ T cells were individually assessed by a panel of 17 immune surface markers identifying different T cell subsets and underlying the T cell repertoire diversity. Transcriptional signature was assessed by NanoString technology.
Results
Phenotypic signature identified a higher proportion of exhausted CD57+CD39+ cytotoxic CD8+ cells in CAR T products of non-responders (NR) in comparison to pts with complete response (CR). Furthermore, CD8+ CAR T cells of NR pts were characterized by CD28 and IL-7R loss, and lower levels of CCR7. Expression of T cell checkpoint inhibitor PD1 was high in these exhausted CD8+ cells. Importantly, higher proportion of exhausted cytotoxic CD8+ cells was detected in PB of NR comparing to responders, suggesting the association of CAR T exhaustion with a dysfunctional phenotype of pts’ T cells. In contrast, CD4+ cells demonstrated low frequency of exhausted phenotype both in the PB as well as the CAR T product from both NR and CR pts, indicating that CD4+ T cells are less prone to exhaustion compared to their CD8+ counterparts, suggesting better persistence of CD4 CAR T cells following antigen exposure.
The transcriptional signature of the CAR T products overlaid with the phenotypic characterization. Higher levels of IL-7R, CD7, BATF3, IL15, FOS, MYC, CD69 and CD27 genes were detected in the CAR T cells of CR pts. In contrast, the CAR T cells of resistant pts were enriched with terminally exhausted population, upregulating CD57, TOX, EOMES, GZMB, GZMA, GZMH, CCL4 and LAG3 genes.
Finally, the functional capacity of the CAR T cells was evaluated in co-culture experiments with CD19+ Raji cells. Stimulation with CD19+ targets induced proliferation and cytokine secretion of the CAR T effectors. Importantly, reduced proliferation and reduced IL-2 secretion correlated with the exhaustion signature. Of note, CAR T cells with exhausted phenotype demonstrated killing capabilities comparable with the non-exhausted CAR cells. Similarly, CAR T cell degranulation, a prerequisite for target cell lysis did not differ as well. These data suggest that the in vitro cytolytic activity of exhausted CAR T cells may be preserved while their proliferation and cytokine production is clinically insufficient.
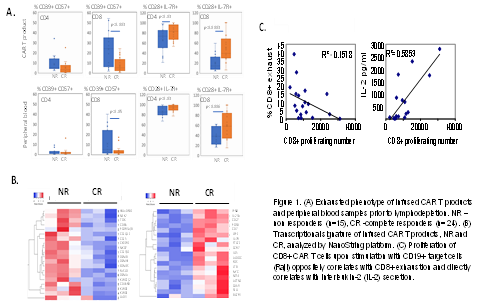
Conclusion
Overall, these results reveal potential cellular and molecular mediators of resistance, identifying an enrichment of terminally exhausted CD8+ T cells as a main feature observed in PB and CAR T products of resistant patients. Delineating these mechanisms may guide future T cells engineering studies to enhance the efficacy and durability of CAR T therapy in B cell malignancies.
Keyword(s): CAR-T, Molecular markers, Phenotype, Resistance
Abstract: EP716
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Biology & Translational Research
Background
In spite of high rates of overall response following chimeric antigen receptor (CAR) T cell therapy its curative potential is jeopardized by exhaustion of the CAR T cells conceivably mediated by the immunosuppressive tumor microenvironment and excessive antigen exposure. However, cellular and molecular predictors of clinical response are not fully elucidated.
Aims
To identify phenotypic, transcriptional and cellular mediators of resistance to CAR T immune therapy.
Methods
We performed a broad immunophenotypic and transcriptomic analysis of the T cells from patients (pts) with relapsed/refractory B cell malignancies (n-39: NHL-28, ALL-5), and CLL-6) treated with locally produced CD19 CAR T cells in correlation with pts’ day 28 clinical response. Intact CD3+ T cells that were obtained from peripheral blood (PB) samples pre Flu/Cy lymphodepletion using negative magnetic sorting and the manufactured CAR T products were analyzed. The CD8+ and CD4+ T cells were individually assessed by a panel of 17 immune surface markers identifying different T cell subsets and underlying the T cell repertoire diversity. Transcriptional signature was assessed by NanoString technology.
Results
Phenotypic signature identified a higher proportion of exhausted CD57+CD39+ cytotoxic CD8+ cells in CAR T products of non-responders (NR) in comparison to pts with complete response (CR). Furthermore, CD8+ CAR T cells of NR pts were characterized by CD28 and IL-7R loss, and lower levels of CCR7. Expression of T cell checkpoint inhibitor PD1 was high in these exhausted CD8+ cells. Importantly, higher proportion of exhausted cytotoxic CD8+ cells was detected in PB of NR comparing to responders, suggesting the association of CAR T exhaustion with a dysfunctional phenotype of pts’ T cells. In contrast, CD4+ cells demonstrated low frequency of exhausted phenotype both in the PB as well as the CAR T product from both NR and CR pts, indicating that CD4+ T cells are less prone to exhaustion compared to their CD8+ counterparts, suggesting better persistence of CD4 CAR T cells following antigen exposure.
The transcriptional signature of the CAR T products overlaid with the phenotypic characterization. Higher levels of IL-7R, CD7, BATF3, IL15, FOS, MYC, CD69 and CD27 genes were detected in the CAR T cells of CR pts. In contrast, the CAR T cells of resistant pts were enriched with terminally exhausted population, upregulating CD57, TOX, EOMES, GZMB, GZMA, GZMH, CCL4 and LAG3 genes.
Finally, the functional capacity of the CAR T cells was evaluated in co-culture experiments with CD19+ Raji cells. Stimulation with CD19+ targets induced proliferation and cytokine secretion of the CAR T effectors. Importantly, reduced proliferation and reduced IL-2 secretion correlated with the exhaustion signature. Of note, CAR T cells with exhausted phenotype demonstrated killing capabilities comparable with the non-exhausted CAR cells. Similarly, CAR T cell degranulation, a prerequisite for target cell lysis did not differ as well. These data suggest that the in vitro cytolytic activity of exhausted CAR T cells may be preserved while their proliferation and cytokine production is clinically insufficient.

Conclusion
Overall, these results reveal potential cellular and molecular mediators of resistance, identifying an enrichment of terminally exhausted CD8+ T cells as a main feature observed in PB and CAR T products of resistant patients. Delineating these mechanisms may guide future T cells engineering studies to enhance the efficacy and durability of CAR T therapy in B cell malignancies.
Keyword(s): CAR-T, Molecular markers, Phenotype, Resistance


