
Contributions
Abstract: EP709
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
CAD is a rare and chronic autoimmune hemolytic anemia driven by activation of the classical complement pathway. Complement activation increases vascular inflammatory markers via generation of anaphylotoxin C3a- and C5a-mediated response of increased cytokine production (eg, interleukins [IL]-6 and IL-10) (Cofiell et al. Blood. 2015; Landsem et al. Clin Exp Immunol. 2013). In CAD, classical complement pathway activation and resulting chronic inflammation may contribute to patient fatigue beyond anemia. A chronic complement-mediated proinflammatory state exists in other hemolytic diseases such as paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome (Cofiell et al. Blood. 2015; Weitz et al. Thromb Res. 2012) with cytokine levels associated with fatigue (Weitz et al. Thromb Res. 2012; Montoya et al. Proc Natl Acad Sci USA. 2017); however, this association has not been formally studied in CAD. Sutimlimab (formerly BIVV009) is a first-in-class humanized immunoglobulin G4 monoclonal anti-C1s antibody that selectively inhibits the classical complement pathway. Here we summarize the results of an analysis of inflammatory cytokine expression and fatigue over the Part A 26-week treatment period of CARDINAL.
Aims
To assess key inflammatory cytokine IL-6 and IL-10 activity and their relation to fatigue in patients with CAD and the effect of treatment with sutimlimab.
Methods
Twenty-four patients with CAD and recent history of transfusion were enrolled in CARDINAL Part A and received intravenous sutimlimab on Days 0 and 7, then biweekly infusions (6.5 g dose for weight <75 kg [n=17]; 7.5 g dose for weight ≥75 kg [n=7]). Cytokine profiles (IL-6 and IL-10) and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores were assessed from baseline to Weeks 1, 3, 5, 9, 13, and 25 as represented by the treatment assessment time point after initiating sutimlimab treatment. Summary statistics were reported at each time point.
Results
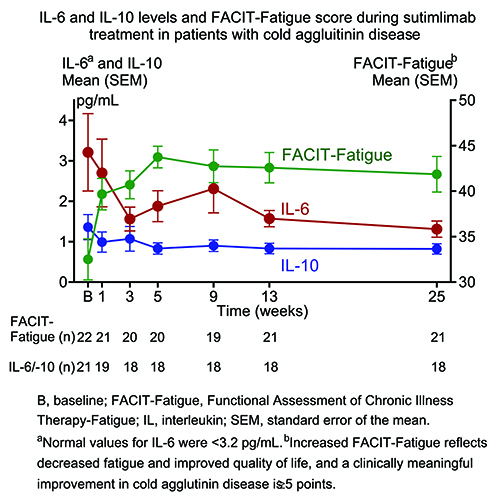
In CARDINAL Part A, baseline mean FACIT-Fatigue score (mean pg/mL [standard error of the mean (SEM)]) was 32.5 (2.3), which is comparable to fatigue severity in another complement-mediated hemolytic anemia, paroxysmal nocturnal hemoglobinuria (Schrezenmeier et al. Haematologica. 2014), and cancer (Escalente et al. Cancer Med. 2019). Sutimlimab treatment rapidly inhibited the classical complement pathway with a rapid improvement in mean FACIT-Fatigue score by Week 1 that was maintained through to the treatment assessment time point (Figure). Mean IL-6 level was 3.21 pg/mL at baseline, decreased as early as Week 1 (2.70 pg/mL) after starting sutimlimab treatment and was 1.31 pg/mL at Week 25 (Figure). A similar pattern of change was observed for IL-10, with the mean values of 1.36 pg/mL at baseline and 0.82 pg/mL at Week 25 (Figure). Decreased inflammation, as demonstrated by IL-6 and IL-10 activity, inversely correlated with FACIT-Fatigue score improvements over time.
Conclusion
Sutimlimab treatment in the CARDINAL Part A study was associated with rapid and steady decrease in IL-6 and IL-10 levels that coincided with clinically meaningful improvements in fatigue. These results suggest that, in addition to anemia, complement-mediated inflammation may contribute to fatigue manifestation in patients with CAD and improve with complement inhibition. Furthermore, they highlight the influence of classical complement pathway inhibition on inflammatory activity in CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Cytokine, Fatigue, Inflammation
Abstract: EP709
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
CAD is a rare and chronic autoimmune hemolytic anemia driven by activation of the classical complement pathway. Complement activation increases vascular inflammatory markers via generation of anaphylotoxin C3a- and C5a-mediated response of increased cytokine production (eg, interleukins [IL]-6 and IL-10) (Cofiell et al. Blood. 2015; Landsem et al. Clin Exp Immunol. 2013). In CAD, classical complement pathway activation and resulting chronic inflammation may contribute to patient fatigue beyond anemia. A chronic complement-mediated proinflammatory state exists in other hemolytic diseases such as paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome (Cofiell et al. Blood. 2015; Weitz et al. Thromb Res. 2012) with cytokine levels associated with fatigue (Weitz et al. Thromb Res. 2012; Montoya et al. Proc Natl Acad Sci USA. 2017); however, this association has not been formally studied in CAD. Sutimlimab (formerly BIVV009) is a first-in-class humanized immunoglobulin G4 monoclonal anti-C1s antibody that selectively inhibits the classical complement pathway. Here we summarize the results of an analysis of inflammatory cytokine expression and fatigue over the Part A 26-week treatment period of CARDINAL.
Aims
To assess key inflammatory cytokine IL-6 and IL-10 activity and their relation to fatigue in patients with CAD and the effect of treatment with sutimlimab.
Methods
Twenty-four patients with CAD and recent history of transfusion were enrolled in CARDINAL Part A and received intravenous sutimlimab on Days 0 and 7, then biweekly infusions (6.5 g dose for weight <75 kg [n=17]; 7.5 g dose for weight ≥75 kg [n=7]). Cytokine profiles (IL-6 and IL-10) and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores were assessed from baseline to Weeks 1, 3, 5, 9, 13, and 25 as represented by the treatment assessment time point after initiating sutimlimab treatment. Summary statistics were reported at each time point.
Results
In CARDINAL Part A, baseline mean FACIT-Fatigue score (mean pg/mL [standard error of the mean (SEM)]) was 32.5 (2.3), which is comparable to fatigue severity in another complement-mediated hemolytic anemia, paroxysmal nocturnal hemoglobinuria (Schrezenmeier et al. Haematologica. 2014), and cancer (Escalente et al. Cancer Med. 2019). Sutimlimab treatment rapidly inhibited the classical complement pathway with a rapid improvement in mean FACIT-Fatigue score by Week 1 that was maintained through to the treatment assessment time point (Figure). Mean IL-6 level was 3.21 pg/mL at baseline, decreased as early as Week 1 (2.70 pg/mL) after starting sutimlimab treatment and was 1.31 pg/mL at Week 25 (Figure). A similar pattern of change was observed for IL-10, with the mean values of 1.36 pg/mL at baseline and 0.82 pg/mL at Week 25 (Figure). Decreased inflammation, as demonstrated by IL-6 and IL-10 activity, inversely correlated with FACIT-Fatigue score improvements over time.

Conclusion
Sutimlimab treatment in the CARDINAL Part A study was associated with rapid and steady decrease in IL-6 and IL-10 levels that coincided with clinically meaningful improvements in fatigue. These results suggest that, in addition to anemia, complement-mediated inflammation may contribute to fatigue manifestation in patients with CAD and improve with complement inhibition. Furthermore, they highlight the influence of classical complement pathway inhibition on inflammatory activity in CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Cytokine, Fatigue, Inflammation


