
Contributions
Abstract: EP706
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
AIHA is a rare acquired disorder resulting from decompensated autoantibody-mediated hemolysis of red blood cells. AIHA is characterized by low hemoglobin levels, which may lead to fatigue, one of the most common symptoms of AIHA. Fatigue has been associated with reduced overall quality of life (QoL) in anemia and other conditions, and a clinically meaningful reduction in anemia-related fatigue (ie, ≥3-point increase in the Functional Assessment of Chronic Illness Therapy–Fatigue subscale [FACIT-F]) may improve QoL. There are no approved treatments for AIHA. Aberrant signaling of PI3Kδ has been associated with autoimmunity. Parsaclisib is an orally administered selective PI3Kδ inhibitor that showed clinical antitumor activity in B-cell malignancies and ameliorated kidney and salivary gland pathology in autoantibody-mediated preclinical disease models of systemic lupus erythematosus and Sjögren’s syndrome, respectively.
Aims
To describe FACIT-F outcomes from an ongoing, multicenter, open-label phase 2 study (NCT03538041) of parsaclisib for the treatment of patients with primary AIHA.
Methods
Eligible patients were aged ≥18 years; were diagnosed with primary warm AIHA (wAIHA), cold agglutinin disease (CAD), or mixed-type AIHA without an underlying lymphoproliferative malignancy or other autoimmune-related underlying conditions; had hemoglobin levels of ≥7–≤10 g/dL; and failed ≥1 prior treatment for AIHA. After informed consent was obtained, patients were enrolled into 1 of 2 successive cohorts and received parsaclisib for 12 weeks. In cohort 1, patients received parsaclisib 1.0 mg once daily (QD) with the option to have a dose increase (2.5 mg QD) at Week 6. In cohort 2, patients received parsaclisib 2.5 mg QD; a dose reduction to 1.0 mg QD was permitted for tolerability. Following completion of the initial 12-week treatment period, eligible patients achieving clinical benefit could continue into an open-label extension period. For the QoL assessment, patients responded to the FACIT-F subscale questionnaire at baseline, Week 6, and Week 12. An increase in FACIT-F score indicates less fatigue and better QoL.
Results
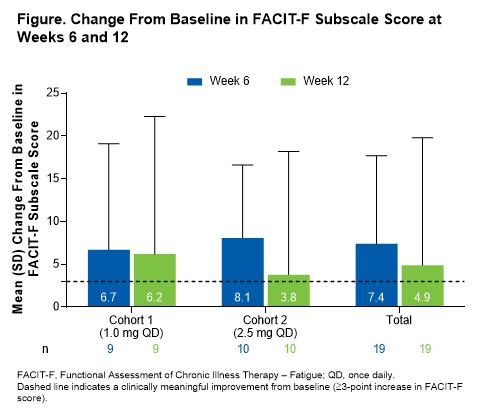
By the data cutoff date (October 12, 2020), 21 patients had enrolled (10 patients in cohort 1; 11 in cohort 2). The 2 cohorts were similar in age (median [range]: cohort 1, 62.0 [46–77] years; cohort 2, 60.0 [22–80] years), sex (male: cohort 1, 40%; cohort 2, 55%), AIHA subtype (wAIHA: cohort 1, 70%; cohort 2, 64%; CAD: cohort 1, 20%; cohort 2, 18%; mixed: cohort 1, 10%; cohort 2, 18%), baseline hemoglobin levels (mean [SD]: cohort 1, 9.1 [0.8] g/dL; cohort 2, 9.0 [0.8] g/dL), and baseline FACIT-F scores (mean [SD]: cohort 1, 32.6 [12.8]; cohort 2, 34.8 [11.7]). Both cohorts showed an increase in FACIT-F subscale score compared with baseline at Week 6 (mean [SD] change from baseline: cohort 1, 6.7 [12.4]; cohort 2, 8.1 [8.5]; total, 7.4 [10.3]) and Week 12 (mean [SD] change from baseline: cohort 1, 6.2 [16.1]; cohort 2, 3.8 [14.4]; total, 4.9 [14.9]; Figure). Mean (SD) percentage change from baseline in FACIT-F score was 40% (71.8) for all patients (cohort 1, 52% [100.7]; cohort 2, 29% [31.8]) at Week 6 and 35% (81.2) for all patients (cohort 1, 55% [111.2]; cohort 2, 17% [37.6]) at Week 12.
Conclusion
Parsaclisib treatment was associated with a clinically meaningful (ie, ≥3-point) improvement in fatigue-related QoL in patients with AIHA. This benefit was seen as early as Week 6 and persisted throughout the treatment period.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Fatigue, PI3K, Quality of life
Abstract: EP706
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
AIHA is a rare acquired disorder resulting from decompensated autoantibody-mediated hemolysis of red blood cells. AIHA is characterized by low hemoglobin levels, which may lead to fatigue, one of the most common symptoms of AIHA. Fatigue has been associated with reduced overall quality of life (QoL) in anemia and other conditions, and a clinically meaningful reduction in anemia-related fatigue (ie, ≥3-point increase in the Functional Assessment of Chronic Illness Therapy–Fatigue subscale [FACIT-F]) may improve QoL. There are no approved treatments for AIHA. Aberrant signaling of PI3Kδ has been associated with autoimmunity. Parsaclisib is an orally administered selective PI3Kδ inhibitor that showed clinical antitumor activity in B-cell malignancies and ameliorated kidney and salivary gland pathology in autoantibody-mediated preclinical disease models of systemic lupus erythematosus and Sjögren’s syndrome, respectively.
Aims
To describe FACIT-F outcomes from an ongoing, multicenter, open-label phase 2 study (NCT03538041) of parsaclisib for the treatment of patients with primary AIHA.
Methods
Eligible patients were aged ≥18 years; were diagnosed with primary warm AIHA (wAIHA), cold agglutinin disease (CAD), or mixed-type AIHA without an underlying lymphoproliferative malignancy or other autoimmune-related underlying conditions; had hemoglobin levels of ≥7–≤10 g/dL; and failed ≥1 prior treatment for AIHA. After informed consent was obtained, patients were enrolled into 1 of 2 successive cohorts and received parsaclisib for 12 weeks. In cohort 1, patients received parsaclisib 1.0 mg once daily (QD) with the option to have a dose increase (2.5 mg QD) at Week 6. In cohort 2, patients received parsaclisib 2.5 mg QD; a dose reduction to 1.0 mg QD was permitted for tolerability. Following completion of the initial 12-week treatment period, eligible patients achieving clinical benefit could continue into an open-label extension period. For the QoL assessment, patients responded to the FACIT-F subscale questionnaire at baseline, Week 6, and Week 12. An increase in FACIT-F score indicates less fatigue and better QoL.
Results
By the data cutoff date (October 12, 2020), 21 patients had enrolled (10 patients in cohort 1; 11 in cohort 2). The 2 cohorts were similar in age (median [range]: cohort 1, 62.0 [46–77] years; cohort 2, 60.0 [22–80] years), sex (male: cohort 1, 40%; cohort 2, 55%), AIHA subtype (wAIHA: cohort 1, 70%; cohort 2, 64%; CAD: cohort 1, 20%; cohort 2, 18%; mixed: cohort 1, 10%; cohort 2, 18%), baseline hemoglobin levels (mean [SD]: cohort 1, 9.1 [0.8] g/dL; cohort 2, 9.0 [0.8] g/dL), and baseline FACIT-F scores (mean [SD]: cohort 1, 32.6 [12.8]; cohort 2, 34.8 [11.7]). Both cohorts showed an increase in FACIT-F subscale score compared with baseline at Week 6 (mean [SD] change from baseline: cohort 1, 6.7 [12.4]; cohort 2, 8.1 [8.5]; total, 7.4 [10.3]) and Week 12 (mean [SD] change from baseline: cohort 1, 6.2 [16.1]; cohort 2, 3.8 [14.4]; total, 4.9 [14.9]; Figure). Mean (SD) percentage change from baseline in FACIT-F score was 40% (71.8) for all patients (cohort 1, 52% [100.7]; cohort 2, 29% [31.8]) at Week 6 and 35% (81.2) for all patients (cohort 1, 55% [111.2]; cohort 2, 17% [37.6]) at Week 12.

Conclusion
Parsaclisib treatment was associated with a clinically meaningful (ie, ≥3-point) improvement in fatigue-related QoL in patients with AIHA. This benefit was seen as early as Week 6 and persisted throughout the treatment period.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Fatigue, PI3K, Quality of life


