
Contributions
Abstract: EP696
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
Hereditary pyruvate kinase (PK) deficiency results in chronic hemolysis that can lead to reduced bone mineral density (BMD). A recent analysis of 159 patients (pts) with PK deficiency showed that >75% of adult pts had osteopenia or osteoporosis at a median age of 34 years (yrs). Mitapivat (AG-348) is an investigational, first-in-class, allosteric activator of PK that has been shown to improve hemoglobin and other hemolysis markers for up to 42 months (mos) in pts with PK deficiency. Mitapivat has mild aromatase inhibition effects; however, it is not clear whether this carries a negative impact on BMD in pts with PK deficiency. Conversely, reducing hemolysis through PKR activation with this agent may have a positive effect on BMD.
Aims
To report BMD over time in pts with PK deficiency receiving long-term treatment with mitapivat in the DRIVE-PK study.
Methods
DRIVE-PK (NCT02476916) is a phase 2, global, randomized, open-label, dose-ranging study in pts ≥18 yrs of age with a confirmed diagnosis of PK deficiency and not receiving regular transfusions (≤3 units of red blood cells ≤12 mos and no transfusions ≤4 mos). Pts were randomized 1:1 to 50 mg or 300 mg of mitapivat twice daily (BID) during a 24-week (wk) core period; eligible pts could enter an extension period to receive mitapivat for up to 8 yrs. Dose adjustments were permitted based on safety and efficacy; following an amendment, doses >25 mg BID were tapered to the lowest effective dose in the extension. BMD was measured using DXA scans at baseline (BL), every 6 mos through month 30, and then annually. DXA changes over time were assessed for pts receiving mitapivat >12 mos. Pts were classified as having normal BMD, osteopenia, or osteoporosis based on DXA T-scores.
Results
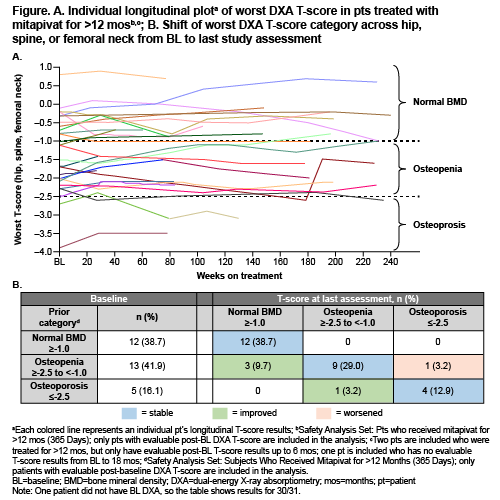
Of 52 participants in DRIVE-PK (median age 34 yrs [range, 18–61]), 31 (32% female) received mitapivat >12 mos and had on-treatment DXA monitoring for up to 56 mos. Of 30 pts who had BL T-scores for total hip, spine, and femoral neck, 12 (38.7%) had a T-score ≥–1.0 (normal BMD) at all locations, 13 (41.9%) had a worst T-score >–2.5 to <–1.0 (osteopenia) at 1 or more locations, and 5 (16.1%) had a worst T-score ≤–2.5 (osteoporosis) at 1 or more locations. As of the latest assessment during the extension period, 12 (38.7%), 11 (35.5%), and 5 (16.1%) of 31 pts had a T-score indicating normal BMD, osteopenia, and osteoporosis, respectively. None of the 12 pts with BMD in the normal range at BL showed worsening of BMD at latest assessment. Of 13 pts with a worst BL T-score of >–2.5 to <–1.0 at 1 or more location(s), 9 remained stable, 1 worsened, and 3 improved to a T-score of ≥–1.0, indicating normal BMD. Of 5 pts with a worst BL T-score of ≤–2.5 at 1 or more location(s), 4 remained stable and 1 improved to a T-score of >–2.5 to <–1.0 (Figure and Table). No fractures were reported during this study.
Conclusion
DXA scanning revealed that BMD was largely stable over time in adult pts with PK deficiency receiving long-term treatment with mitapivat for up to 56 mos, despite a substantial degree of reduced BMD at BL. Therefore, mitapivat does not appear to promote progression of BMD abnormalities in these pts and could halt this process by decreasing hemolysis and ineffective erythropoiesis. Additional BMD data will be collected as part of this ongoing extension study.
Keyword(s): AG-348, Bone mineral density, Osteoporosis, Pyruvate kinase deficiency
Abstract: EP696
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
Hereditary pyruvate kinase (PK) deficiency results in chronic hemolysis that can lead to reduced bone mineral density (BMD). A recent analysis of 159 patients (pts) with PK deficiency showed that >75% of adult pts had osteopenia or osteoporosis at a median age of 34 years (yrs). Mitapivat (AG-348) is an investigational, first-in-class, allosteric activator of PK that has been shown to improve hemoglobin and other hemolysis markers for up to 42 months (mos) in pts with PK deficiency. Mitapivat has mild aromatase inhibition effects; however, it is not clear whether this carries a negative impact on BMD in pts with PK deficiency. Conversely, reducing hemolysis through PKR activation with this agent may have a positive effect on BMD.
Aims
To report BMD over time in pts with PK deficiency receiving long-term treatment with mitapivat in the DRIVE-PK study.
Methods
DRIVE-PK (NCT02476916) is a phase 2, global, randomized, open-label, dose-ranging study in pts ≥18 yrs of age with a confirmed diagnosis of PK deficiency and not receiving regular transfusions (≤3 units of red blood cells ≤12 mos and no transfusions ≤4 mos). Pts were randomized 1:1 to 50 mg or 300 mg of mitapivat twice daily (BID) during a 24-week (wk) core period; eligible pts could enter an extension period to receive mitapivat for up to 8 yrs. Dose adjustments were permitted based on safety and efficacy; following an amendment, doses >25 mg BID were tapered to the lowest effective dose in the extension. BMD was measured using DXA scans at baseline (BL), every 6 mos through month 30, and then annually. DXA changes over time were assessed for pts receiving mitapivat >12 mos. Pts were classified as having normal BMD, osteopenia, or osteoporosis based on DXA T-scores.
Results
Of 52 participants in DRIVE-PK (median age 34 yrs [range, 18–61]), 31 (32% female) received mitapivat >12 mos and had on-treatment DXA monitoring for up to 56 mos. Of 30 pts who had BL T-scores for total hip, spine, and femoral neck, 12 (38.7%) had a T-score ≥–1.0 (normal BMD) at all locations, 13 (41.9%) had a worst T-score >–2.5 to <–1.0 (osteopenia) at 1 or more locations, and 5 (16.1%) had a worst T-score ≤–2.5 (osteoporosis) at 1 or more locations. As of the latest assessment during the extension period, 12 (38.7%), 11 (35.5%), and 5 (16.1%) of 31 pts had a T-score indicating normal BMD, osteopenia, and osteoporosis, respectively. None of the 12 pts with BMD in the normal range at BL showed worsening of BMD at latest assessment. Of 13 pts with a worst BL T-score of >–2.5 to <–1.0 at 1 or more location(s), 9 remained stable, 1 worsened, and 3 improved to a T-score of ≥–1.0, indicating normal BMD. Of 5 pts with a worst BL T-score of ≤–2.5 at 1 or more location(s), 4 remained stable and 1 improved to a T-score of >–2.5 to <–1.0 (Figure and Table). No fractures were reported during this study.

Conclusion
DXA scanning revealed that BMD was largely stable over time in adult pts with PK deficiency receiving long-term treatment with mitapivat for up to 56 mos, despite a substantial degree of reduced BMD at BL. Therefore, mitapivat does not appear to promote progression of BMD abnormalities in these pts and could halt this process by decreasing hemolysis and ineffective erythropoiesis. Additional BMD data will be collected as part of this ongoing extension study.
Keyword(s): AG-348, Bone mineral density, Osteoporosis, Pyruvate kinase deficiency


