
Contributions
Abstract: EP689
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
CAD is a rare chronic autoimmune hemolytic anemia characterized by classical complement pathway–mediated hemolysis (predominantly extravascular occurring in the liver), anemia, and fatigue. Sutimlimab (formerly BIVV009) is a first-in-class humanized monoclonal antibody and C1s inhibitor that selectively targets the classical complement pathway, while leaving the lectin and alternative pathways intact.
Aims
CARDINAL (NCT03347396) evaluated sutimlimab efficacy and safety in adults with CAD and a recent transfusion history. One-year interim results of the long-term follow-up are reported.
Methods
CARDINAL is a Phase 3, open-label, single-arm study with a 26-week treatment period (Part A) and ongoing extension (Part B) for 2 years after the last patient finishes Part A. Interim data are available for Part B (data cut: January 16, 2020). Adult patients with confirmed CAD diagnosis, baseline hemoglobin (Hb) ≤10 g/dL, and ≥1 blood transfusion in the past 6 months were enrolled after providing informed consent. Sutimlimab was administered intravenously on Days 0 and 7, then biweekly. Interim Part B analysis assessed long-term efficacy and safety of sutimlimab, and durability of response (including laboratory parameters and quality of life [QOL]). Efficacy endpoints were change from baseline in hemolytic markers and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue Scale. Descriptive statistics, frequency, or percentage were used to analyze outcomes.
Results
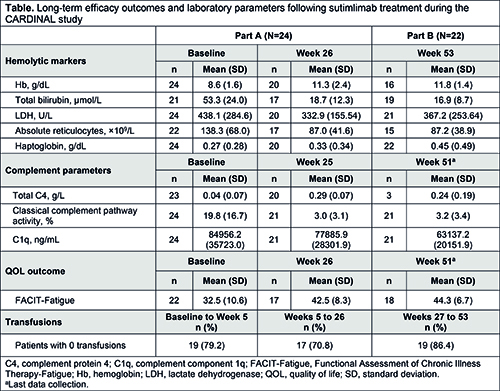
Overall, 24 patients enrolled and 22 finished Part A and entered Part B. Of enrolled patients, 62.5% were female; mean (standard deviation [SD]) age was 71.3 (8.2) years; mean (SD) number of transfusions <6 months before enrollment was 3.2 (4.0); 62.5% had received ≥1 targeted therapy in the last 5 years. Sutimlimab treatment led to rapid improvement in mean Hb level after the first dose; Hb remained >11 g/dL from Weeks 5 (Part A) to 53 (Part B; Table). Mean total bilirubin rapidly normalized by Week 3 and was maintained <20 µmol/L through Week 53. Mean FACIT-Fatigue scores rapidly increased by Week 1 and remained >40 points from Weeks 3 to 51; mean increase from baseline to Week 51 was 11.4, consistent with a clinically meaningful improvement. Improvements in Hb, bilirubin, and FACIT-Fatigue correlated with the normalization of C4 and near-complete inhibition of classical complement pathway activity (by Wieslab assay). Mean C1q levels were unaffected. Normalization of absolute reticulocyte count from baseline to Week 3 was also observed, alongside normalized haptoglobin levels and reductions in lactate dehydrogenase. Of patients who entered Part B, 86.4% were transfusion-free from Weeks 26 to 53. From baseline to the data cutoff date, all 24 patients experienced ≥1 treatment-emergent adverse event (TEAE), with one serious TEAE (viral infection; treatment related). No meningococcal infections or TEAEs of meningitis were reported. One TEAE of device-related thrombosis (unrelated to sutimlimab) was reported in Part B; there were no other vascular thromboembolic TEAEs. No patients developed systemic lupus erythematosus.
Conclusion
Sutimlimab, a first-in-class selective anti-C1s classical complement pathway inhibitor, maintained Hb levels >11g/dL, sustained normalization of bilirubin, and continued to improve FACIT-Fatigue scores, with no newly identified safety concerns at 1 year. These results of the ongoing CARDINAL Part B long-term study demonstrate that continued upstream inhibition of the classical complement pathway with sutimlimab provides sustained and durable treatment effects in chronic CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Clinical trial, Phase III
Abstract: EP689
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
CAD is a rare chronic autoimmune hemolytic anemia characterized by classical complement pathway–mediated hemolysis (predominantly extravascular occurring in the liver), anemia, and fatigue. Sutimlimab (formerly BIVV009) is a first-in-class humanized monoclonal antibody and C1s inhibitor that selectively targets the classical complement pathway, while leaving the lectin and alternative pathways intact.
Aims
CARDINAL (NCT03347396) evaluated sutimlimab efficacy and safety in adults with CAD and a recent transfusion history. One-year interim results of the long-term follow-up are reported.
Methods
CARDINAL is a Phase 3, open-label, single-arm study with a 26-week treatment period (Part A) and ongoing extension (Part B) for 2 years after the last patient finishes Part A. Interim data are available for Part B (data cut: January 16, 2020). Adult patients with confirmed CAD diagnosis, baseline hemoglobin (Hb) ≤10 g/dL, and ≥1 blood transfusion in the past 6 months were enrolled after providing informed consent. Sutimlimab was administered intravenously on Days 0 and 7, then biweekly. Interim Part B analysis assessed long-term efficacy and safety of sutimlimab, and durability of response (including laboratory parameters and quality of life [QOL]). Efficacy endpoints were change from baseline in hemolytic markers and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue Scale. Descriptive statistics, frequency, or percentage were used to analyze outcomes.
Results
Overall, 24 patients enrolled and 22 finished Part A and entered Part B. Of enrolled patients, 62.5% were female; mean (standard deviation [SD]) age was 71.3 (8.2) years; mean (SD) number of transfusions <6 months before enrollment was 3.2 (4.0); 62.5% had received ≥1 targeted therapy in the last 5 years. Sutimlimab treatment led to rapid improvement in mean Hb level after the first dose; Hb remained >11 g/dL from Weeks 5 (Part A) to 53 (Part B; Table). Mean total bilirubin rapidly normalized by Week 3 and was maintained <20 µmol/L through Week 53. Mean FACIT-Fatigue scores rapidly increased by Week 1 and remained >40 points from Weeks 3 to 51; mean increase from baseline to Week 51 was 11.4, consistent with a clinically meaningful improvement. Improvements in Hb, bilirubin, and FACIT-Fatigue correlated with the normalization of C4 and near-complete inhibition of classical complement pathway activity (by Wieslab assay). Mean C1q levels were unaffected. Normalization of absolute reticulocyte count from baseline to Week 3 was also observed, alongside normalized haptoglobin levels and reductions in lactate dehydrogenase. Of patients who entered Part B, 86.4% were transfusion-free from Weeks 26 to 53. From baseline to the data cutoff date, all 24 patients experienced ≥1 treatment-emergent adverse event (TEAE), with one serious TEAE (viral infection; treatment related). No meningococcal infections or TEAEs of meningitis were reported. One TEAE of device-related thrombosis (unrelated to sutimlimab) was reported in Part B; there were no other vascular thromboembolic TEAEs. No patients developed systemic lupus erythematosus.

Conclusion
Sutimlimab, a first-in-class selective anti-C1s classical complement pathway inhibitor, maintained Hb levels >11g/dL, sustained normalization of bilirubin, and continued to improve FACIT-Fatigue scores, with no newly identified safety concerns at 1 year. These results of the ongoing CARDINAL Part B long-term study demonstrate that continued upstream inhibition of the classical complement pathway with sutimlimab provides sustained and durable treatment effects in chronic CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Clinical trial, Phase III


