
Contributions
Abstract: EP686
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
Pyruvate kinase deficiency (PKD) is an ultra-rare chronic hereditary hemolytic anemia of variable severity, ranging from mild anemia to life-long transfusion dependence. Conventional treatment consisting of transfusions and splenectomy is challenged by clinical trials of PK activators (e.g. Mitapivat) and lentiviral gene therapy. Mild cases are often not monitored for long-term complications resulting from permanent haemolysis. In addition, lack of standardized diagnostic procedures combined with a low prevalence results in a significant number of undiagnosed or misdiagnosed patients. Consequently, this hampers access to proper treatment options for PKD patients in Europe.
Aims
The aim of this study was to assess the distribution of PKD patients in Europe in order to improve diagnosis and follow-up rates and to facilitate access to available treatment options by patients suffering from PKD.
Methods
The study was conducted through RADeep, which is endorsed by ERN-EuroBloodNet. Up to 145 centres dealing with RADs from 16 European countries were included in the study. Data on PKD patients in follow-up was requested, stratified by age range, adult and paediatric (<18) and by severity according to two main variables: splenectomy and regular transfusion, which was defined as >6 transfusions per year. Data on number of diagnosis in the last 25 years was requested only from diagnostic centres. Data was correlated to a country’s total population (2020). Based on highest frequency, estimations were calculated for the expected number of PKD patients in all participating countries.
Results
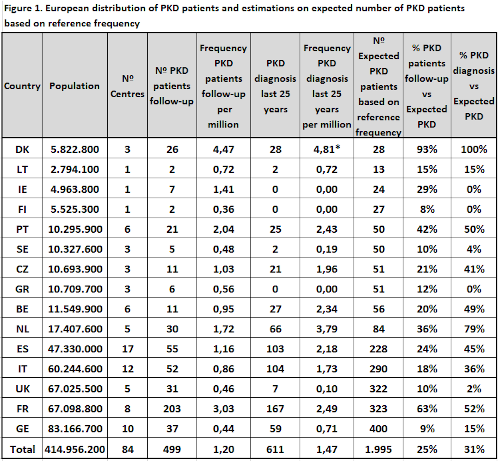
Data on 499 PKD patients in follow-up and 611 diagnosed PKD patients retrieved from 84 medical centres from 15 European countries is shown on figure 1. Highest frequency for both PKD patients in follow-up and PKD diagnosis was found in Denmark, respectively 4,47 and 4,81 per million. This is in line with previous published data on PKD prevalence, with estimations ranging between 3,2 and 8,5 per million. Based on the PKD diagnosis frequency in Denmark, we calculated the expected number of PKD patients in the rest of participating countries. Results showed an expected number of PKD patients of 1.995, meaning that our study is covering on average 25% of PKD patients in follow-up and 31% of diagnosed PKD cases. Important differences on PKD patients in follow-up coverage are found among countries, from 63% in France to less than 25% in most of participating countries. Diagnostic coverage was highest in the Netherlands, France and Portugal. According to stratification of PKD patients based on severity, 267 adult patients were stratified as follows; 18% splenectomised on regular transfusion, 4% non-splenectomised on regular transfusion, 43% splenectomised on occasional or non-transfusion and 35% non-splenectomised on occasional or non-transfusion. Finally, % of PKD patients genotyped was found to be on average 90% and 86% for adults and pediatrics respectively (range 20-100%).
Conclusion
This study clearly shows the need to improve and standardize PKD patients’ pathway at the national level across Europe in order to ensure adequate and timely diagnosis, and proper access to best treatment for any PKD patient. National recognition of centres of expertise for PKD for follow-up (i.e. France), centralized diagnosis (i.e. Netherlands) and/or existing registries (i.e. France, Netherlands, Denmark) dramatically improve disease coverage. Therefore, important efforts are needed in most other European countries to improve PKD diagnosis and follow-up rates.
Keyword(s): Diagnosis, Epidemiology, Pyruvate kinase deficiency
Abstract: EP686
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
Pyruvate kinase deficiency (PKD) is an ultra-rare chronic hereditary hemolytic anemia of variable severity, ranging from mild anemia to life-long transfusion dependence. Conventional treatment consisting of transfusions and splenectomy is challenged by clinical trials of PK activators (e.g. Mitapivat) and lentiviral gene therapy. Mild cases are often not monitored for long-term complications resulting from permanent haemolysis. In addition, lack of standardized diagnostic procedures combined with a low prevalence results in a significant number of undiagnosed or misdiagnosed patients. Consequently, this hampers access to proper treatment options for PKD patients in Europe.
Aims
The aim of this study was to assess the distribution of PKD patients in Europe in order to improve diagnosis and follow-up rates and to facilitate access to available treatment options by patients suffering from PKD.
Methods
The study was conducted through RADeep, which is endorsed by ERN-EuroBloodNet. Up to 145 centres dealing with RADs from 16 European countries were included in the study. Data on PKD patients in follow-up was requested, stratified by age range, adult and paediatric (<18) and by severity according to two main variables: splenectomy and regular transfusion, which was defined as >6 transfusions per year. Data on number of diagnosis in the last 25 years was requested only from diagnostic centres. Data was correlated to a country’s total population (2020). Based on highest frequency, estimations were calculated for the expected number of PKD patients in all participating countries.
Results
Data on 499 PKD patients in follow-up and 611 diagnosed PKD patients retrieved from 84 medical centres from 15 European countries is shown on figure 1. Highest frequency for both PKD patients in follow-up and PKD diagnosis was found in Denmark, respectively 4,47 and 4,81 per million. This is in line with previous published data on PKD prevalence, with estimations ranging between 3,2 and 8,5 per million. Based on the PKD diagnosis frequency in Denmark, we calculated the expected number of PKD patients in the rest of participating countries. Results showed an expected number of PKD patients of 1.995, meaning that our study is covering on average 25% of PKD patients in follow-up and 31% of diagnosed PKD cases. Important differences on PKD patients in follow-up coverage are found among countries, from 63% in France to less than 25% in most of participating countries. Diagnostic coverage was highest in the Netherlands, France and Portugal. According to stratification of PKD patients based on severity, 267 adult patients were stratified as follows; 18% splenectomised on regular transfusion, 4% non-splenectomised on regular transfusion, 43% splenectomised on occasional or non-transfusion and 35% non-splenectomised on occasional or non-transfusion. Finally, % of PKD patients genotyped was found to be on average 90% and 86% for adults and pediatrics respectively (range 20-100%).

Conclusion
This study clearly shows the need to improve and standardize PKD patients’ pathway at the national level across Europe in order to ensure adequate and timely diagnosis, and proper access to best treatment for any PKD patient. National recognition of centres of expertise for PKD for follow-up (i.e. France), centralized diagnosis (i.e. Netherlands) and/or existing registries (i.e. France, Netherlands, Denmark) dramatically improve disease coverage. Therefore, important efforts are needed in most other European countries to improve PKD diagnosis and follow-up rates.
Keyword(s): Diagnosis, Epidemiology, Pyruvate kinase deficiency


