
Contributions
Abstract: EP685
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
There are no approved therapies for AIHA, a rare acquired condition caused by autoantibody-mediated decompensated destruction of red blood cells. The phosphatidylinositol 3-kinase δ inhibitor parsaclisib has shown efficacy in preclinical models of autoantibody-mediated diseases.
Aims
To describe preliminary 12-week efficacy and safety from an ongoing, multicenter, open-label phase 2 trial (NCT03538041) of parsaclisib in patients with primary AIHA.
Methods
Eligible patients were aged ≥18 years; had a diagnosis of warm AIHA (wAIHA), cold agglutinin disease (CAD), or mixed-type AIHA without an underlying lymphoproliferative malignancy; hemoglobin (Hgb) ≥7–≤10 g/dL; and failure of ≥1 standard therapy. Concomitant use of low-dose corticosteroids (≤20 mg/d prednisone equivalent) was permitted. After providing informed consent, patients received parsaclisib for 12 weeks at a starting oral dose of 1.0 mg once daily (QD; cohort 1) or 2.5 mg QD (cohort 2; enrolled after ≥6 patients with wAIHA in cohort 1 completed 6 weeks). Patients in cohort 1 could have their dose increased to 2.5 mg QD after 6 weeks if they had not achieved meaningful clinical response and/or required transfusions; those in cohort 2 could have dose reductions to 1.0 mg QD for tolerability. Patients achieving clinical benefit could continue to receive parsaclisib in an extension period. Primary endpoints were efficacy (proportion of patients with complete response [CR; Hgb ≥12 g/dL] or partial response [PR; Hgb 10–12 g/dL or ≥2 g/dL increase from baseline] at any visit from Week 6–12) and safety (treatment-emergent adverse events [TEAEs]).
Results
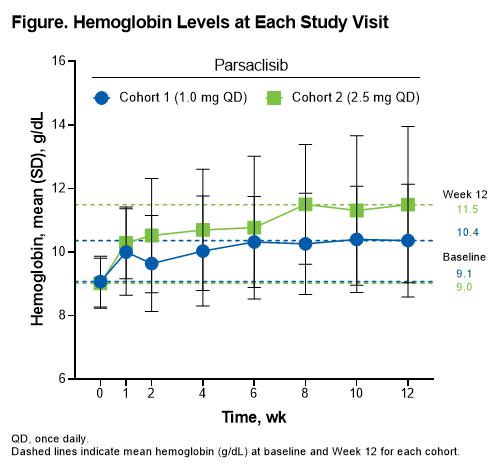
A total of 21 patients (cohort 1, n=10 [8 patients had a dose increase]; cohort 2, n=11) were enrolled and received parsaclisib; 17 patients (81%) completed 12 weeks of treatment. Mean (SD) age was 58.8 (16.6) years; 11 patients (52%) were female and 20 (95%) were White. Fourteen (67%), 4 (19%), and 3 (14%) patients had wAIHA, CAD, and mixed AIHA, respectively, and 6 (29%) had transfusions in the past year. Mean (SD) Hgb at baseline was 9.0 (0.8) g/dL and was similar between cohorts. Overall, 7 patients (33%) achieved CR (cohort 1, n=2 [20%]; cohort 2, n=5 [45%]) and 14 (67%) had PR (cohort 1, n=6 [60%]; cohort 2, n=8 [73%]) at any visit from Week 6 to 12. Dose-dependent increases from baseline in Hgb were observed over time, with cohort 2 (2.5 mg QD) achieving mean Hgb >10 g/dL by Week 2 (Figure). Other hemolytic markers including haptoglobin, reticulocytes, indirect bilirubin, and lactate dehydrogenase trended toward normalization. TEAEs occurred in 18 patients (86%). Five patients (24%) had grade ≥3 TEAEs, with only neutropenia occurring in >1 patient (n=2 [10%]). One patient (5%) had a serious TEAE (hemolytic anemia; deemed unrelated to treatment). Treatment-related AEs (TRAEs) were reported in 6 patients (29%); pruritic rash was the only TRAE occurring in >1 patient (n=2 [10%]), and neutropenia and thrombocytopenia were the only grade ≥3 TRAEs (n=1 [5%] each). TEAEs led to dose interruption in 4 patients (19%) and included neutropenia (n=2 [10%]), diarrhea (n=1 [5%]), and hepatotoxicity (n=1 [5%]; deemed unrelated to parsaclisib). One patient (5%) in cohort 1 discontinued parsaclisib due to a TEAE (thrombocytopenia). There were no fatal TEAEs.
Conclusion
Parsaclisib was generally well tolerated, and treatment resulted in durable normalization of Hgb levels as early as Week 2. Parsaclisib has the potential to be an effective oral treatment for both wAIHA and CAD and warrants further clinical investigation.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Clinical trial, Hemoglobin, PI3K
Abstract: EP685
Type: E-Poster Presentation
Session title: Enzymopathies, membranopathies and other anemias
Background
There are no approved therapies for AIHA, a rare acquired condition caused by autoantibody-mediated decompensated destruction of red blood cells. The phosphatidylinositol 3-kinase δ inhibitor parsaclisib has shown efficacy in preclinical models of autoantibody-mediated diseases.
Aims
To describe preliminary 12-week efficacy and safety from an ongoing, multicenter, open-label phase 2 trial (NCT03538041) of parsaclisib in patients with primary AIHA.
Methods
Eligible patients were aged ≥18 years; had a diagnosis of warm AIHA (wAIHA), cold agglutinin disease (CAD), or mixed-type AIHA without an underlying lymphoproliferative malignancy; hemoglobin (Hgb) ≥7–≤10 g/dL; and failure of ≥1 standard therapy. Concomitant use of low-dose corticosteroids (≤20 mg/d prednisone equivalent) was permitted. After providing informed consent, patients received parsaclisib for 12 weeks at a starting oral dose of 1.0 mg once daily (QD; cohort 1) or 2.5 mg QD (cohort 2; enrolled after ≥6 patients with wAIHA in cohort 1 completed 6 weeks). Patients in cohort 1 could have their dose increased to 2.5 mg QD after 6 weeks if they had not achieved meaningful clinical response and/or required transfusions; those in cohort 2 could have dose reductions to 1.0 mg QD for tolerability. Patients achieving clinical benefit could continue to receive parsaclisib in an extension period. Primary endpoints were efficacy (proportion of patients with complete response [CR; Hgb ≥12 g/dL] or partial response [PR; Hgb 10–12 g/dL or ≥2 g/dL increase from baseline] at any visit from Week 6–12) and safety (treatment-emergent adverse events [TEAEs]).
Results
A total of 21 patients (cohort 1, n=10 [8 patients had a dose increase]; cohort 2, n=11) were enrolled and received parsaclisib; 17 patients (81%) completed 12 weeks of treatment. Mean (SD) age was 58.8 (16.6) years; 11 patients (52%) were female and 20 (95%) were White. Fourteen (67%), 4 (19%), and 3 (14%) patients had wAIHA, CAD, and mixed AIHA, respectively, and 6 (29%) had transfusions in the past year. Mean (SD) Hgb at baseline was 9.0 (0.8) g/dL and was similar between cohorts. Overall, 7 patients (33%) achieved CR (cohort 1, n=2 [20%]; cohort 2, n=5 [45%]) and 14 (67%) had PR (cohort 1, n=6 [60%]; cohort 2, n=8 [73%]) at any visit from Week 6 to 12. Dose-dependent increases from baseline in Hgb were observed over time, with cohort 2 (2.5 mg QD) achieving mean Hgb >10 g/dL by Week 2 (Figure). Other hemolytic markers including haptoglobin, reticulocytes, indirect bilirubin, and lactate dehydrogenase trended toward normalization. TEAEs occurred in 18 patients (86%). Five patients (24%) had grade ≥3 TEAEs, with only neutropenia occurring in >1 patient (n=2 [10%]). One patient (5%) had a serious TEAE (hemolytic anemia; deemed unrelated to treatment). Treatment-related AEs (TRAEs) were reported in 6 patients (29%); pruritic rash was the only TRAE occurring in >1 patient (n=2 [10%]), and neutropenia and thrombocytopenia were the only grade ≥3 TRAEs (n=1 [5%] each). TEAEs led to dose interruption in 4 patients (19%) and included neutropenia (n=2 [10%]), diarrhea (n=1 [5%]), and hepatotoxicity (n=1 [5%]; deemed unrelated to parsaclisib). One patient (5%) in cohort 1 discontinued parsaclisib due to a TEAE (thrombocytopenia). There were no fatal TEAEs.

Conclusion
Parsaclisib was generally well tolerated, and treatment resulted in durable normalization of Hgb levels as early as Week 2. Parsaclisib has the potential to be an effective oral treatment for both wAIHA and CAD and warrants further clinical investigation.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Clinical trial, Hemoglobin, PI3K


