
Contributions
Abstract: EP684
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Bosutinib is approved for the treatment of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) resistant/intolerant to prior therapy and newly diagnosed Ph+ chronic phase (CP) CML. Body mass index (BMI) was shown to influence treatment response with front-line dasatinib vs imatinib.
Aims
We report the efficacy and safety of bosutinib and imatinib by BMI in patients with newly diagnosed CP CML.
Methods
In the open-label BFORE trial (NCT02130557), patients were randomized to receive 400 mg once daily bosutinib or imatinib. Outcomes were assessed according to baseline BMI ≥25 or <25 kg/m2. This post hoc analysis was based on the final 5-y analysis (database lock: June 12, 2020).
Results
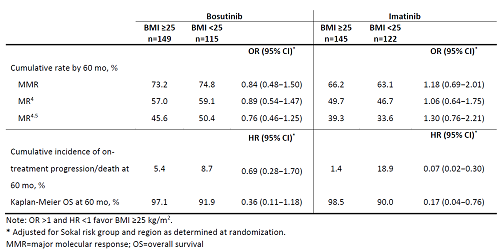
In the bosutinib and imatinib arms, respectively, 149 (56.4%) vs 115 (43.6%) patients and 145 (54.3%) vs 122 (45.7%) patients had BMI ≥25 vs <25. In both the bosutinib and imatinib arms, median Treatment duration and time on study was 55 mo for patients with BMI ≥25 or <25; respective median dose intensity was 394 vs 393 mg/d and 400 vs 400 mg/d. Molecular response (MR) rates are shown in the table. Cumulative incidence of major MR was similar in patients with ≥25 vs <25 receiving bosutinib (HR 0.99; 95% CI 0.74−1.31) or imatinib (HR 1.09; 95% CI 0.81−1.47). Event-free survival and overall survival rates at 60 mo are shown in the table. Most common reasons for treatment discontinuation were adverse events (AEs) (bosutinib 28.2 vs 20.0%; imatinib 13.3 vs 10.7%) and lack of efficacy (bosutinib 5.4 vs 5.2%; imatinib 16.1 vs 19.8%). In patients with BMI ≥25 vs <25, dose reductions and interruptions due to treatment-emergent AEs (TEAEs) occurred in 43.6 % vs 46.2% and 66.4% vs 69.7% of patients with bosutinib and 24.5% vs 24.6% and 40.6% vs 50.8% with imatinib. Any grade TEAEs in ≥30% of patients with BMI ≥25 vs <25 were diarrhea (73.8 vs 73.1%), nausea (40.9 vs 31.9%), thrombocytopenia (30.9 vs 41.2%), increased alanine (37.6 vs 28.6%) and aspartate aminotransferase (30.2 vs 20.2%) with bosutinib and diarrhea (49.0 vs 29.5%), nausea (46.2 vs 37.7%), muscle spasms (33.6 vs 26.2%), neutropenia (14.7 vs 32.0%) and thrombocytopenia (10.5% vs 30.3%) with imatinib.
Conclusion
Efficacy of bosutinib was consistent in patients with BMI ≥25 or <25; however, with imatinib a low (vs high) BMI appeared to be associated with worse survival outcomes. Differences in certain TEAEs were observed between BMI subgroups in both treatment arms.
Keyword(s): Chronic myeloid leukemia, Clinical trial, Tyrosine kinase inhibitor
Abstract: EP684
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Bosutinib is approved for the treatment of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) resistant/intolerant to prior therapy and newly diagnosed Ph+ chronic phase (CP) CML. Body mass index (BMI) was shown to influence treatment response with front-line dasatinib vs imatinib.
Aims
We report the efficacy and safety of bosutinib and imatinib by BMI in patients with newly diagnosed CP CML.
Methods
In the open-label BFORE trial (NCT02130557), patients were randomized to receive 400 mg once daily bosutinib or imatinib. Outcomes were assessed according to baseline BMI ≥25 or <25 kg/m2. This post hoc analysis was based on the final 5-y analysis (database lock: June 12, 2020).
Results
In the bosutinib and imatinib arms, respectively, 149 (56.4%) vs 115 (43.6%) patients and 145 (54.3%) vs 122 (45.7%) patients had BMI ≥25 vs <25. In both the bosutinib and imatinib arms, median Treatment duration and time on study was 55 mo for patients with BMI ≥25 or <25; respective median dose intensity was 394 vs 393 mg/d and 400 vs 400 mg/d. Molecular response (MR) rates are shown in the table. Cumulative incidence of major MR was similar in patients with ≥25 vs <25 receiving bosutinib (HR 0.99; 95% CI 0.74−1.31) or imatinib (HR 1.09; 95% CI 0.81−1.47). Event-free survival and overall survival rates at 60 mo are shown in the table. Most common reasons for treatment discontinuation were adverse events (AEs) (bosutinib 28.2 vs 20.0%; imatinib 13.3 vs 10.7%) and lack of efficacy (bosutinib 5.4 vs 5.2%; imatinib 16.1 vs 19.8%). In patients with BMI ≥25 vs <25, dose reductions and interruptions due to treatment-emergent AEs (TEAEs) occurred in 43.6 % vs 46.2% and 66.4% vs 69.7% of patients with bosutinib and 24.5% vs 24.6% and 40.6% vs 50.8% with imatinib. Any grade TEAEs in ≥30% of patients with BMI ≥25 vs <25 were diarrhea (73.8 vs 73.1%), nausea (40.9 vs 31.9%), thrombocytopenia (30.9 vs 41.2%), increased alanine (37.6 vs 28.6%) and aspartate aminotransferase (30.2 vs 20.2%) with bosutinib and diarrhea (49.0 vs 29.5%), nausea (46.2 vs 37.7%), muscle spasms (33.6 vs 26.2%), neutropenia (14.7 vs 32.0%) and thrombocytopenia (10.5% vs 30.3%) with imatinib.

Conclusion
Efficacy of bosutinib was consistent in patients with BMI ≥25 or <25; however, with imatinib a low (vs high) BMI appeared to be associated with worse survival outcomes. Differences in certain TEAEs were observed between BMI subgroups in both treatment arms.
Keyword(s): Chronic myeloid leukemia, Clinical trial, Tyrosine kinase inhibitor


