Contributions
Abstract: EP682
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
A survey of healthcare professionals in the UK to describe current pathways and management practices in chronic myeloid leukaemia:the ADAPT CML survey.
Aims
To understand the current diagnosis (Dx), treatment (Tx) and management, including unmet need, landscape for UK patients (pts) with chronic phase chronic myeloid leukaemia (CML-CP).
Methods
We conducted a survey with 46 Haematology consultants (27 from district general hospitals [DGH]; 19 from tertiary centres [TC]). Structured interviews with Medical Science Liaisons from Novartis, were conducted between July and October 2020. Responses were descriptively analysed.
Results
Most (70%) respondents saw 5-10 newly diagnosed CML-CP pts per year (43%, 39%, 17% had a current total CML-CP pts population estimate of ≤50, >50 ≤100, and >100 respectively). All respondents stated they routinely use physical examination, white blood counts, platelet counts, haemoglobin counts and blood film in their CML diagnostic work-up, although only 89% used chromosome banding analysis (CBA). Information on cardiovascular (CV) risk most commonly captured routinely in pt notes prior to tyrosine kinase inhibitor (TKI) initiation were comorbidities (98%), smoking status (98%) and CV history (91%), while total cholesterol (61%) and blood glucose (52%) were less frequently captured. Only 32% used QRisk assessment. Sokal remained the most commonly used prognostic scoring tool (76%).
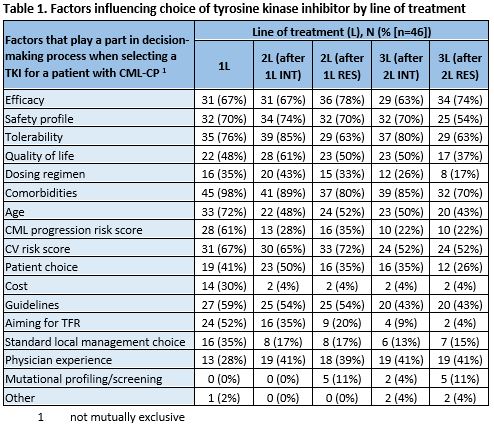
The median (interquartile range) estimated percentage of pts on different lines of treatment (LoT) at DGH were 1st line (1L): 66% (55%–75%); 2nd line (2L): 25% (20%–32.5%); 3rd line (3L): 10% (5%–10%). At TC, 1L: 57.5% (50%–61.5%); 2L: 30% (21.25%–30%); 3L: 16.5% (10%–20%). Regardless of LoT or reason for prior TKI discontinuation (intolerance [INT]/resistance [RES]), major molecular response was the main response goal in 1L, 2L and 3L. INT was the most cited reason for switching to 2L (67%) or 3L (54%) TKI, while switch to 4L was RES (80%). Impact on quality of life (QoL) (85%) and tolerability affecting compliance (80%) were the most cited definitions of TKI INT. The most commonly reported choice of TKI was imatinib (98%) for 1L, dasatinib (50%) or nilotinib (41%) for 2L and a 2nd generation TKI (39%), TKI based on mutation profile (37%), or bosutinib (22%) for 3L. Additional factors influencing TKI choice at each LoT (not mutually exclusive) are listed in Table 1. In the first and subsequent years of TKI use, all respondents reported frequent monitoring of response by RQ-PCR or cytogenetics (78% using BCR-ABL1 RQ-PCR only); in line with ELN recommendations.
Most respondents (70%) used ELN2020 to guide patient management. Increased frequency of monitoring (65%) and evaluation of compliance (65%) were the most cited actions following an ELN warning response. For pts with >1 warning response or a failure response, a switch in therapy (72%/93%, respectively) and mutation analysis (76%/89%, respectively) were the most cited actions taken. Most frequently cited unmet needs in CML per line were improving QoL (1L, 48%), TKI toxicity (2L, 63%) and RES to current options (3L,72%).
Conclusion
The survey suggests TKI tolerability remains an unmet need in both 1L and 2L CML treatment while TKI resistance is more a concern in later lines. The survey also suggests management of CML in UK is broadly in line with ELN recommendations. However, it also highlights areas for improvement for optimal pt management including the need for well documented CV risk assessment, CBA in all diagnostic workup, mutation analysis in all warning/failure, and increased evaluation of TKI compliance.
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Tyrosine kinase inhibitor
Abstract: EP682
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
A survey of healthcare professionals in the UK to describe current pathways and management practices in chronic myeloid leukaemia:the ADAPT CML survey.
Aims
To understand the current diagnosis (Dx), treatment (Tx) and management, including unmet need, landscape for UK patients (pts) with chronic phase chronic myeloid leukaemia (CML-CP).
Methods
We conducted a survey with 46 Haematology consultants (27 from district general hospitals [DGH]; 19 from tertiary centres [TC]). Structured interviews with Medical Science Liaisons from Novartis, were conducted between July and October 2020. Responses were descriptively analysed.
Results
Most (70%) respondents saw 5-10 newly diagnosed CML-CP pts per year (43%, 39%, 17% had a current total CML-CP pts population estimate of ≤50, >50 ≤100, and >100 respectively). All respondents stated they routinely use physical examination, white blood counts, platelet counts, haemoglobin counts and blood film in their CML diagnostic work-up, although only 89% used chromosome banding analysis (CBA). Information on cardiovascular (CV) risk most commonly captured routinely in pt notes prior to tyrosine kinase inhibitor (TKI) initiation were comorbidities (98%), smoking status (98%) and CV history (91%), while total cholesterol (61%) and blood glucose (52%) were less frequently captured. Only 32% used QRisk assessment. Sokal remained the most commonly used prognostic scoring tool (76%).
The median (interquartile range) estimated percentage of pts on different lines of treatment (LoT) at DGH were 1st line (1L): 66% (55%–75%); 2nd line (2L): 25% (20%–32.5%); 3rd line (3L): 10% (5%–10%). At TC, 1L: 57.5% (50%–61.5%); 2L: 30% (21.25%–30%); 3L: 16.5% (10%–20%). Regardless of LoT or reason for prior TKI discontinuation (intolerance [INT]/resistance [RES]), major molecular response was the main response goal in 1L, 2L and 3L. INT was the most cited reason for switching to 2L (67%) or 3L (54%) TKI, while switch to 4L was RES (80%). Impact on quality of life (QoL) (85%) and tolerability affecting compliance (80%) were the most cited definitions of TKI INT. The most commonly reported choice of TKI was imatinib (98%) for 1L, dasatinib (50%) or nilotinib (41%) for 2L and a 2nd generation TKI (39%), TKI based on mutation profile (37%), or bosutinib (22%) for 3L. Additional factors influencing TKI choice at each LoT (not mutually exclusive) are listed in Table 1. In the first and subsequent years of TKI use, all respondents reported frequent monitoring of response by RQ-PCR or cytogenetics (78% using BCR-ABL1 RQ-PCR only); in line with ELN recommendations.
Most respondents (70%) used ELN2020 to guide patient management. Increased frequency of monitoring (65%) and evaluation of compliance (65%) were the most cited actions following an ELN warning response. For pts with >1 warning response or a failure response, a switch in therapy (72%/93%, respectively) and mutation analysis (76%/89%, respectively) were the most cited actions taken. Most frequently cited unmet needs in CML per line were improving QoL (1L, 48%), TKI toxicity (2L, 63%) and RES to current options (3L,72%).
Conclusion
The survey suggests TKI tolerability remains an unmet need in both 1L and 2L CML treatment while TKI resistance is more a concern in later lines. The survey also suggests management of CML in UK is broadly in line with ELN recommendations. However, it also highlights areas for improvement for optimal pt management including the need for well documented CV risk assessment, CBA in all diagnostic workup, mutation analysis in all warning/failure, and increased evaluation of TKI compliance.
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Tyrosine kinase inhibitor