
Contributions
Abstract: EP681
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
TKI dose reduction may be safely used for AE correction or prevention in pts with optimal response. The feasibility of TKI dose reduction as a step before treatment-free remission (TFR) phase has been confirmed in several trials. However, no unified scheme has been developed for each TKI. The connection of plasma blood TKI concentration with the AE and response preservation has not been studied.
Aims
To provide the first interim results of the «Study of REduction And DIscontinuation Treatment of TKI (READIT)» (NCT 04578847): evaluation of the response rates and first data of imatinib (IM) plasma blood concentrations after dose reduction.
Methods
Two consecutive phases are planned in the trial: 1) TKI dose reduction phase for at least 12 months (mo), 2) TFR observation (24 mo). The TKI dose reduction phase consists of two 6 mo steps: 1st step (300 mg for IM, 400 mg for nilotinib (NIL), 50 mg for dasatinib (DAS) and 300 mg for bosutinib (BOS)) and 2nd step (25 mg for DAS and 200 mg for IM, NIL and BOS) of TKI dose reduction. Inclusion criteria: adult CML in chronic phase (CP), TKI therapy > 3 years, major molecular response (MMR, BCR-ABL1 ≤0,1%) >2 years and deep molecular response (DMR, BCR-ABL1 ≤0.01%) >1 year. Recruitment of patients in reduced doses was possible, provided that the duration of the current therapy is at least 6 mo. Treatment by TKI doses of previous step was resumed in case of confirmed MMR loss (BCR-ABL>0.1% in two samples).
Plasma blood concentration was measured for IM (24±2 hours (Cmin) and 4 hours (Cmax) after drug intake). We used the Mann-Whitney U-test for comparative analysis.
Results
A total of 70 CML pts were included from Dec.2019 till March 2021. The baseline characteristics: male 41%, Median (Me) age 56 years (range 23-74); Me TKI therapy duration 6,3 years (range 2.5-18.9); Me duration of MMR and DMR was 3,3 years (range 2-11,2) and 2 y (range 1-11,2), respectively. At baseline 46 (66%) pts received IM (39 pts-400 mg, 3 pts–300 mg, 4 pts–200 mg) and 24 (34%) pts - second-generation (2G) TKI: NIL - 13 pts (6 pts-800-600 mg, 6 pts-400 mg, 1 pt–200 mg), DAS- 6 pts (4 pts-100-70 mg, 2 pts–50 mg), BOS- 5 pts (1 pt–500 mg, 2 pts–300 mg, 2 pts–200 mg).
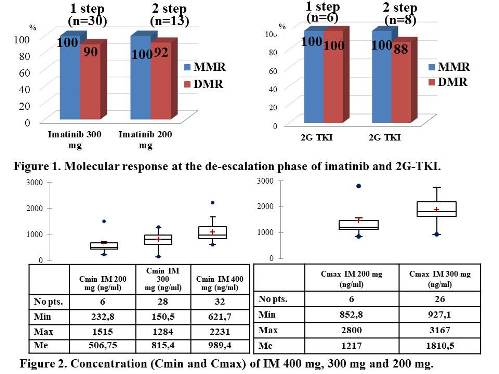
Thirty-six pts have completed 1st dose reduction step. Three pts on IM lost DMR (1 pt-after 3 mo, 2 pts-after 6 mo dose reduction), no cases of DMR loss in the 2G TKI group. Twenty-one pts completed 2nd step of dose reduction. Two pts lost DMR after 3 mo: 1 pt on IM and 1 pt on DAS. (Fig.1). Of those 5 pts 4 were restored DMR at the same dose. There were no cases of confirmed loss of MMR during the dose reduction phase.
We analyzed the IM concentration in 98 plasma blood samples (Cmax-in 32, Cmin–in 66). There was a significant difference in Cmin of IM 400 mg, 300 mg and 200 mg (p=0.0019) and no difference in Cmax of IM 300 mg and 200 mg. (p=0.078) (Fig.2).
No difference in IM concentration (Cmin and Cmax on 300 and 200 mg) was in 5 pts who lost DMR compared to pts without DMR loss. No significant difference IM 400 mg Cmin was in pts with and without AE (p=0.26).
Conclusion
No MMR loss was detected during therapy with 1st and 2nd step of TKI dose reduction and about 90% of pts remained in DMR. This proves the safety of TKI treatment at reduced doses. The results will be updated within a longer follow-up in order to evaluate the impact of de-escalation phase on TFR rates. The role of different TKI concentrations will be studied.
Keyword(s): Chronic myeloid leukemia, Clinical trial, Pharmacokinetic, Tyrosine kinase inhibitor
Abstract: EP681
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
TKI dose reduction may be safely used for AE correction or prevention in pts with optimal response. The feasibility of TKI dose reduction as a step before treatment-free remission (TFR) phase has been confirmed in several trials. However, no unified scheme has been developed for each TKI. The connection of plasma blood TKI concentration with the AE and response preservation has not been studied.
Aims
To provide the first interim results of the «Study of REduction And DIscontinuation Treatment of TKI (READIT)» (NCT 04578847): evaluation of the response rates and first data of imatinib (IM) plasma blood concentrations after dose reduction.
Methods
Two consecutive phases are planned in the trial: 1) TKI dose reduction phase for at least 12 months (mo), 2) TFR observation (24 mo). The TKI dose reduction phase consists of two 6 mo steps: 1st step (300 mg for IM, 400 mg for nilotinib (NIL), 50 mg for dasatinib (DAS) and 300 mg for bosutinib (BOS)) and 2nd step (25 mg for DAS and 200 mg for IM, NIL and BOS) of TKI dose reduction. Inclusion criteria: adult CML in chronic phase (CP), TKI therapy > 3 years, major molecular response (MMR, BCR-ABL1 ≤0,1%) >2 years and deep molecular response (DMR, BCR-ABL1 ≤0.01%) >1 year. Recruitment of patients in reduced doses was possible, provided that the duration of the current therapy is at least 6 mo. Treatment by TKI doses of previous step was resumed in case of confirmed MMR loss (BCR-ABL>0.1% in two samples).
Plasma blood concentration was measured for IM (24±2 hours (Cmin) and 4 hours (Cmax) after drug intake). We used the Mann-Whitney U-test for comparative analysis.
Results
A total of 70 CML pts were included from Dec.2019 till March 2021. The baseline characteristics: male 41%, Median (Me) age 56 years (range 23-74); Me TKI therapy duration 6,3 years (range 2.5-18.9); Me duration of MMR and DMR was 3,3 years (range 2-11,2) and 2 y (range 1-11,2), respectively. At baseline 46 (66%) pts received IM (39 pts-400 mg, 3 pts–300 mg, 4 pts–200 mg) and 24 (34%) pts - second-generation (2G) TKI: NIL - 13 pts (6 pts-800-600 mg, 6 pts-400 mg, 1 pt–200 mg), DAS- 6 pts (4 pts-100-70 mg, 2 pts–50 mg), BOS- 5 pts (1 pt–500 mg, 2 pts–300 mg, 2 pts–200 mg).
Thirty-six pts have completed 1st dose reduction step. Three pts on IM lost DMR (1 pt-after 3 mo, 2 pts-after 6 mo dose reduction), no cases of DMR loss in the 2G TKI group. Twenty-one pts completed 2nd step of dose reduction. Two pts lost DMR after 3 mo: 1 pt on IM and 1 pt on DAS. (Fig.1). Of those 5 pts 4 were restored DMR at the same dose. There were no cases of confirmed loss of MMR during the dose reduction phase.
We analyzed the IM concentration in 98 plasma blood samples (Cmax-in 32, Cmin–in 66). There was a significant difference in Cmin of IM 400 mg, 300 mg and 200 mg (p=0.0019) and no difference in Cmax of IM 300 mg and 200 mg. (p=0.078) (Fig.2).
No difference in IM concentration (Cmin and Cmax on 300 and 200 mg) was in 5 pts who lost DMR compared to pts without DMR loss. No significant difference IM 400 mg Cmin was in pts with and without AE (p=0.26).

Conclusion
No MMR loss was detected during therapy with 1st and 2nd step of TKI dose reduction and about 90% of pts remained in DMR. This proves the safety of TKI treatment at reduced doses. The results will be updated within a longer follow-up in order to evaluate the impact of de-escalation phase on TFR rates. The role of different TKI concentrations will be studied.
Keyword(s): Chronic myeloid leukemia, Clinical trial, Pharmacokinetic, Tyrosine kinase inhibitor


