
Contributions
Abstract: EP679
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Ponatinib is a 3rd generation tyrosine kinase inhibitor (TKI) indicated in chronic phase (CP), accelerated phase (AP) and blast phase (BP) CML as well as in patients (pts) with T315I mutation.
Aims
TOPASE is an observational cohort, designed to evaluate, with a 2 to 4 years follow-up period, the efficacy and safety of ponatinib in routine clinical practice.
Methods
This interim analysis reports results from data of pts included in 42 CML French centers from February 2018 to December 2020.
Results
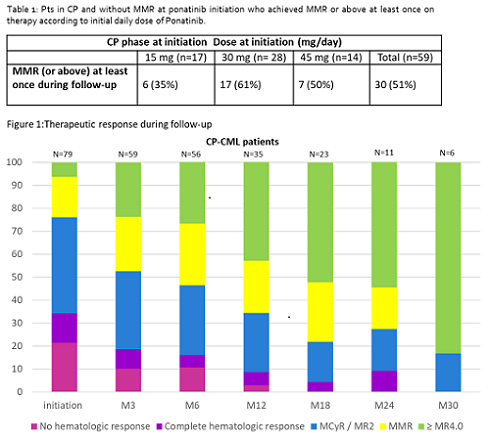
110 pts were registered, 97 in CP and 13 in AP/BP; median age was 58.7 [20-90] years. Reason for prescription was resistance in 73% and intolerance in 26% of pts. Ponatinib was prescribed in 14 % of cases in second line and in 53% in third line of treatment. Last treatment administered prior to ponatinib was dasatinib (39%), bosutinib (24%), nilotinib (24%), imatinib (10%) and ABL001 (2%). Twenty seven of the 69 pts screened for mutations harbored a BCR-ABL mutation (T315I in 13). Thirty two per cent had an history of hypertension and 30% were treated for hypertension at inclusion. In CP-CML pts, ponatinib starting dose was 15mg/d in 37%, 30 mg/d in 43% and 45 mg/d in 20%. Respectively in each subgroup the percentage of intolerant pts were: 42, 24 and 10 %.Considering AP/BP pts, ponatinib initiating dose was 45 mg/d in 62%, 30 mg/d in 23% and 15 mg/d in 15% of pts. At the initiation of ponatinib, out of 93 CP-CML pts evaluable for response, 26% of pts were in MMR or beyond, 39% were in MCyR and/ or MR2, 14% were in CHR and 21% were not in CHR. Efficacy analysis was performed on all the 59 CP-CML pts with BCR-ABL1 > 0.1% at ponatinib start and with at least one evaluation under treatment, as defined in the protocol.After a median treatment duration of 10.7 months [0.4; 36.4], MMR was achieved in 30 pts (51%) (Table 1). Overall, the global responses in all evaluable patients showed a shift towards deeper responses up to 30 months (Table 2). In 37 pts (34%) [31 in CP and 6 in AP/BP] ponatinib was discontinued early after a median exposure of 5.8 months. Reasons for discontinuation were lack of response or progression (n=15), adverse event (n=14) or another reason (n=8). Treatment related adverse events (TRAE) were reported in 52% of pts (n=52) (serious = 11% [n=11]). Most common TRAE (all/ serious) were hypertension (9/1), constipation (5/1), dry skin (5/0), lipase increase (4/0).Cardiovascular events (5/4) included acute coronary syndrome (1), angina pectoris (1), aortic insufficiency (1), atrial fibrillation (1), pericardial effusion (1) and cerebrovascular events (2/2) included ischemic stroke and cerebrovascular accident. Four pts died during follow-up: 1 from disease progression (pt included in AP/BP) and 3 from AE (1 general status alteration, 1 pancytopenia [pt included in AP/BP] and 1 from pulmonary embolism, the only AE considered as related to ponatinib). More complete data analysis on the incidence of TRAEs according to the dose of ponatinib are currently conducted and will be presented.
Conclusion
Results from this real-world French cohort confirm the high efficiency of ponatinib in TKI-resistant and intolerant CML pts. Ponatinib induces deep molecular responses, with an acceptable safety profile. Vascular events rate seems low in this study, which is the largest prospective observational study on ponatinib use in Europe.
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Targeted therapy, Tyrosine kinase inhibitor
Abstract: EP679
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Ponatinib is a 3rd generation tyrosine kinase inhibitor (TKI) indicated in chronic phase (CP), accelerated phase (AP) and blast phase (BP) CML as well as in patients (pts) with T315I mutation.
Aims
TOPASE is an observational cohort, designed to evaluate, with a 2 to 4 years follow-up period, the efficacy and safety of ponatinib in routine clinical practice.
Methods
This interim analysis reports results from data of pts included in 42 CML French centers from February 2018 to December 2020.
Results
110 pts were registered, 97 in CP and 13 in AP/BP; median age was 58.7 [20-90] years. Reason for prescription was resistance in 73% and intolerance in 26% of pts. Ponatinib was prescribed in 14 % of cases in second line and in 53% in third line of treatment. Last treatment administered prior to ponatinib was dasatinib (39%), bosutinib (24%), nilotinib (24%), imatinib (10%) and ABL001 (2%). Twenty seven of the 69 pts screened for mutations harbored a BCR-ABL mutation (T315I in 13). Thirty two per cent had an history of hypertension and 30% were treated for hypertension at inclusion. In CP-CML pts, ponatinib starting dose was 15mg/d in 37%, 30 mg/d in 43% and 45 mg/d in 20%. Respectively in each subgroup the percentage of intolerant pts were: 42, 24 and 10 %.Considering AP/BP pts, ponatinib initiating dose was 45 mg/d in 62%, 30 mg/d in 23% and 15 mg/d in 15% of pts. At the initiation of ponatinib, out of 93 CP-CML pts evaluable for response, 26% of pts were in MMR or beyond, 39% were in MCyR and/ or MR2, 14% were in CHR and 21% were not in CHR. Efficacy analysis was performed on all the 59 CP-CML pts with BCR-ABL1 > 0.1% at ponatinib start and with at least one evaluation under treatment, as defined in the protocol.After a median treatment duration of 10.7 months [0.4; 36.4], MMR was achieved in 30 pts (51%) (Table 1). Overall, the global responses in all evaluable patients showed a shift towards deeper responses up to 30 months (Table 2). In 37 pts (34%) [31 in CP and 6 in AP/BP] ponatinib was discontinued early after a median exposure of 5.8 months. Reasons for discontinuation were lack of response or progression (n=15), adverse event (n=14) or another reason (n=8). Treatment related adverse events (TRAE) were reported in 52% of pts (n=52) (serious = 11% [n=11]). Most common TRAE (all/ serious) were hypertension (9/1), constipation (5/1), dry skin (5/0), lipase increase (4/0).Cardiovascular events (5/4) included acute coronary syndrome (1), angina pectoris (1), aortic insufficiency (1), atrial fibrillation (1), pericardial effusion (1) and cerebrovascular events (2/2) included ischemic stroke and cerebrovascular accident. Four pts died during follow-up: 1 from disease progression (pt included in AP/BP) and 3 from AE (1 general status alteration, 1 pancytopenia [pt included in AP/BP] and 1 from pulmonary embolism, the only AE considered as related to ponatinib). More complete data analysis on the incidence of TRAEs according to the dose of ponatinib are currently conducted and will be presented.

Conclusion
Results from this real-world French cohort confirm the high efficiency of ponatinib in TKI-resistant and intolerant CML pts. Ponatinib induces deep molecular responses, with an acceptable safety profile. Vascular events rate seems low in this study, which is the largest prospective observational study on ponatinib use in Europe.
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Targeted therapy, Tyrosine kinase inhibitor


