
Contributions
Abstract: EP676
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
The natural course of untreated or TKI-resistant chronic myeloid leukemia (CML) is progression to a blast phase (BP), which may be myeloid (MBP) or lymphoid. Over the past 20 years, the advent of tyrosine kinase inhibitors (TKIs) against the BCR-ABL1 fusion protein has dramatically reduced the incidence of CML-BP. However, CML-BP still occurs in some patients, and outcomes remain exceedingly poor with no consensus frontline treatment approach.
Aims
We retrospectively evaluated the outcomes with different frontline treatment approaches in patients with CML-MBP who were treated at our institution over 2 decades.
Methods
Patients were included if they were diagnosed with CML-MBP as defined by >20% bone marrow myeloblasts (WHO criteria) between 2000-2019, with or without the presence of concomitant extramedullary (EM) disease, and received frontline therapy for CML-MBP. Patients with EM-only disease were excluded. Patients could have received prior therapy for CML in chronic phase (CP) or accelerated phase (AP) but no prior therapy for BP aside from hydroxyurea. Patients were divided into 4 frontline treatment approaches: 1) intensive chemotherapy (IC)+TKI, 2) hypomethylating agent (HMA)+TKI, 3) TKI alone, or 4) IC alone. We assessed morphologic/hematologic responses per AML IWG 2003 criteria and cytogenetic/molecular responses per CML ELN 2020 criteria. We assessed cumulative incidence of relapse (CIR), event-free survival (EFS), overall survival (OS), and the benefit of allogeneic stem cell transplantation (ASCT).
Results
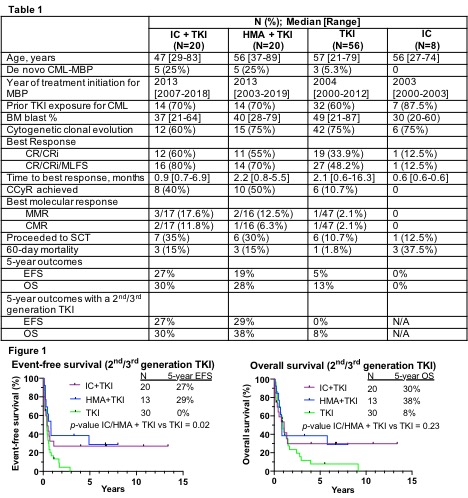
104 patients were identified who received frontline treatment for CML-MBP (IC+TKI, n=20; HMA+TKI, n=20; TKI alone, n=56; IC alone, n=8) . Baseline patient characteristics are shown in Table 1. Patients who received IC+TKI were younger than other groups. The majority of patients in each group had received at least one prior TKI for treatment of CML CP/AP. A 2nd/3rd generation TKI was used for CML-MBP in 100% of patients treated with IC+TKI, 65% with HMA+TKI, and 54% with TKI alone.
Hematologic, cytogenetic and molecular response rates are shown in Table 1. Given similarities in outcomes between IC+TKI and HMA+TKI, these two groups were combined for analysis (IC/HMA+TKI, n=40). Compared to TKI alone, IC/HMA+TKI resulted in a higher CR/CRi rate (57.5% vs 33.9%, p<0.05), higher complete cytogenetic response (CCyR) rate (45% vs 10.7%, p<0.001), and led to more patients proceeding to ASCT (32.5% vs 10.7%, p<0.01). These superior outcomes with IC/HMA+TKI were observed despite a higher 60-day mortality with combination therapy compared to TKI alone (15% vs 1.8%, p<0.05).
With a median follow-up of 6.7 years, long-term outcomes were similar between the IC+TKI and HMA+TKI groups, and both were superior to TKI alone when all patients were considered as well as when analysis was limited to those treated with a 2nd/3rd generation TKI (Figure 1). When using a 2nd/ 3rd generation TKI, IC/HMA+TKI led to improved 5-year CIR (44% vs 86%, p<0.05), 5-year EFS (28% vs 0%, p<0.05), and 5-year OS (34% vs 8%, p=0.23) compared to TKI alone.
Landmark analysis was performed to assess the benefit of ASCT in patients who achieved an objective response (CR/CRi/MLFS). Median time to ASCT was 4.7 months, and long-term survival was superior for patients who proceeded to ASCT (58% vs 22%, p=0.12) compared to those who did not proceed to ASCT at the landmark timepoint.
Conclusion
Compared to patients treated with TKI alone for CML-MBP, treatment with IC+TKI or HMA+TKI leads to improved response rates, CIR, EFS, and OS, particularly for patients treated with a 2nd/3rd generation TKI.
Keyword(s): CML blast crisis
Abstract: EP676
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
The natural course of untreated or TKI-resistant chronic myeloid leukemia (CML) is progression to a blast phase (BP), which may be myeloid (MBP) or lymphoid. Over the past 20 years, the advent of tyrosine kinase inhibitors (TKIs) against the BCR-ABL1 fusion protein has dramatically reduced the incidence of CML-BP. However, CML-BP still occurs in some patients, and outcomes remain exceedingly poor with no consensus frontline treatment approach.
Aims
We retrospectively evaluated the outcomes with different frontline treatment approaches in patients with CML-MBP who were treated at our institution over 2 decades.
Methods
Patients were included if they were diagnosed with CML-MBP as defined by >20% bone marrow myeloblasts (WHO criteria) between 2000-2019, with or without the presence of concomitant extramedullary (EM) disease, and received frontline therapy for CML-MBP. Patients with EM-only disease were excluded. Patients could have received prior therapy for CML in chronic phase (CP) or accelerated phase (AP) but no prior therapy for BP aside from hydroxyurea. Patients were divided into 4 frontline treatment approaches: 1) intensive chemotherapy (IC)+TKI, 2) hypomethylating agent (HMA)+TKI, 3) TKI alone, or 4) IC alone. We assessed morphologic/hematologic responses per AML IWG 2003 criteria and cytogenetic/molecular responses per CML ELN 2020 criteria. We assessed cumulative incidence of relapse (CIR), event-free survival (EFS), overall survival (OS), and the benefit of allogeneic stem cell transplantation (ASCT).
Results
104 patients were identified who received frontline treatment for CML-MBP (IC+TKI, n=20; HMA+TKI, n=20; TKI alone, n=56; IC alone, n=8) . Baseline patient characteristics are shown in Table 1. Patients who received IC+TKI were younger than other groups. The majority of patients in each group had received at least one prior TKI for treatment of CML CP/AP. A 2nd/3rd generation TKI was used for CML-MBP in 100% of patients treated with IC+TKI, 65% with HMA+TKI, and 54% with TKI alone.
Hematologic, cytogenetic and molecular response rates are shown in Table 1. Given similarities in outcomes between IC+TKI and HMA+TKI, these two groups were combined for analysis (IC/HMA+TKI, n=40). Compared to TKI alone, IC/HMA+TKI resulted in a higher CR/CRi rate (57.5% vs 33.9%, p<0.05), higher complete cytogenetic response (CCyR) rate (45% vs 10.7%, p<0.001), and led to more patients proceeding to ASCT (32.5% vs 10.7%, p<0.01). These superior outcomes with IC/HMA+TKI were observed despite a higher 60-day mortality with combination therapy compared to TKI alone (15% vs 1.8%, p<0.05).
With a median follow-up of 6.7 years, long-term outcomes were similar between the IC+TKI and HMA+TKI groups, and both were superior to TKI alone when all patients were considered as well as when analysis was limited to those treated with a 2nd/3rd generation TKI (Figure 1). When using a 2nd/ 3rd generation TKI, IC/HMA+TKI led to improved 5-year CIR (44% vs 86%, p<0.05), 5-year EFS (28% vs 0%, p<0.05), and 5-year OS (34% vs 8%, p=0.23) compared to TKI alone.
Landmark analysis was performed to assess the benefit of ASCT in patients who achieved an objective response (CR/CRi/MLFS). Median time to ASCT was 4.7 months, and long-term survival was superior for patients who proceeded to ASCT (58% vs 22%, p=0.12) compared to those who did not proceed to ASCT at the landmark timepoint.

Conclusion
Compared to patients treated with TKI alone for CML-MBP, treatment with IC+TKI or HMA+TKI leads to improved response rates, CIR, EFS, and OS, particularly for patients treated with a 2nd/3rd generation TKI.
Keyword(s): CML blast crisis


