
Contributions
Abstract: EP674
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Due to the challenge of COVID-19 pandemic the prognosis for patients (pts) with hematologic (hem) tumors, including chronic myeloid leukemia (CML), was difficult to make. With the data accumulation and vaccination start in early 2021, a view of the problem begins to change.
Aims
We aimed to describe the disease course of COVID-19, treatment and first vaccination data in pts with CML in Russian Federation.
Methods
A total of 113 CML pts were analyzed: 106 pts with lab-confirmed or suspected COVID-19 and 7 pts vaccinated against COVID-19. Registration of COVID-19 cases was done prospectively during in person or remote consultations from March 2020 till February 2021. The pts were participants of CHRONOS19, a nationwide observational cohort study of adult (≥18 y) pts with wide spectrum of hem diseases (NCT04422470). We performed a sub-analysis for CML and COVID-19 cohort with the data of 3 hem clinics regarding diagnostics, disease course, treatment and outcomes. Additionally, we evaluated the tolerability and first vaccination results in 7 CML pts (Sputnik V in civil vaccination).
Results
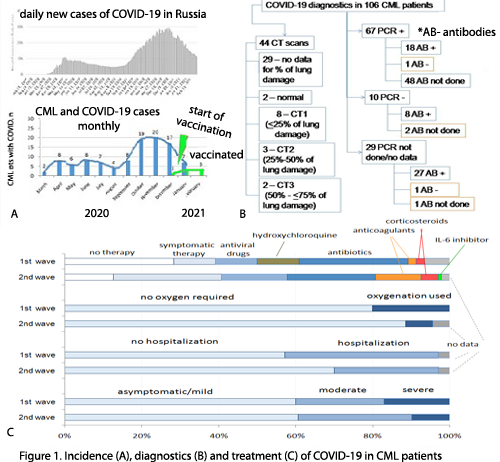
Case registration corresponded to incidence in the country with clear 2 “waves”: 1st one in March-August (n=35) and 2nd starting from September 2020 (n=71) (fig1). COVID-19 was clinically and/or lab diagnosed and treated according to current national recommendations. CML was newly diagnosed during COVID-19 in 8(8%) pts, while 86(81%) were on treatment and 12(11%) were in treatment-free remission (TFR). One case was in pregnant pt. CML phases were chronic(CP)/accelerated/blast crisis in 95(89,6%), 6(5,4%) and 5(5%) pts respectively. Median (Me) age of pts was 52 years (range 22-75), 58(55%) were male. No CML therapy during COVID-19 was in 34 (32%) pts (interrupted or not started), therapy was held in 67 (63%), no data in 5 (5%). Disease course of COVID-19 was asymptomatic, mild, moderate and severe in 5 (5%), 59 (56%), 29(27%) and 13(12%) pts respectively. No therapy for COVID-19 was in 30 (28%) pts, symptomatic therapy (fever relief, anti-cough, decongestants, vitamins etc) - in 73 (69%) pts, antiviral drugs and antibiotics were used in 28 (26%) and 44 (42%) pts respectively. Anticoagulants, corticosteroids and IL-6 inhibitors were introduced mainly during 2nd wave while hydroxychloroquine was abandoned (fig.1). Hospitalization was in 33(31%) pts, oxygenation was applied in 12 (11%) pts. The outcomes were as follows: 101(95%) pts alive (98 recovered, 3 not yet), 4 (3,7%) pts died (2 due to COVID, 2 to CML progression), 1(1,3%) –no data. No COVID reinfection was detected so far. No factors connected with moderate/severe vs asymptomatic/mild COVID-19 were found when analyzing gender, CML phase, TFR, comorbidities, 1st and 2nd wave, except age>52 years (p=0,039). A trend of mild disease was in pts taking TKI vs without TKI (p=0,065).
Seven CP CML pts with Me age 63 years (range 50-70) were vaccinated against COVID-19 since December 2020: 2 had the 1st shot and 5 pts completed vaccination. All 7 pts tolerated the procedure well, with no adverse effects. One pt was checked for antibodies 21 days after the 2nd shot and revealed a high level of anti-SARS-CoV-2 IgG with coefficient of positivity 6,2 (reference range 0-0,9).
Conclusion
The incidence, disease course and COVID-19 dependent mortality rates in CML pts seem to be similar to common population. Older age is a factor of moderate/severe disease course. The TKI impact on COVID-19 course and post-vaccination immunity in CML pts need to be studied.
Keyword(s): Chronic myeloid leukemia, COVID-19, Tyrosine kinase inhibitor
Abstract: EP674
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Due to the challenge of COVID-19 pandemic the prognosis for patients (pts) with hematologic (hem) tumors, including chronic myeloid leukemia (CML), was difficult to make. With the data accumulation and vaccination start in early 2021, a view of the problem begins to change.
Aims
We aimed to describe the disease course of COVID-19, treatment and first vaccination data in pts with CML in Russian Federation.
Methods
A total of 113 CML pts were analyzed: 106 pts with lab-confirmed or suspected COVID-19 and 7 pts vaccinated against COVID-19. Registration of COVID-19 cases was done prospectively during in person or remote consultations from March 2020 till February 2021. The pts were participants of CHRONOS19, a nationwide observational cohort study of adult (≥18 y) pts with wide spectrum of hem diseases (NCT04422470). We performed a sub-analysis for CML and COVID-19 cohort with the data of 3 hem clinics regarding diagnostics, disease course, treatment and outcomes. Additionally, we evaluated the tolerability and first vaccination results in 7 CML pts (Sputnik V in civil vaccination).
Results
Case registration corresponded to incidence in the country with clear 2 “waves”: 1st one in March-August (n=35) and 2nd starting from September 2020 (n=71) (fig1). COVID-19 was clinically and/or lab diagnosed and treated according to current national recommendations. CML was newly diagnosed during COVID-19 in 8(8%) pts, while 86(81%) were on treatment and 12(11%) were in treatment-free remission (TFR). One case was in pregnant pt. CML phases were chronic(CP)/accelerated/blast crisis in 95(89,6%), 6(5,4%) and 5(5%) pts respectively. Median (Me) age of pts was 52 years (range 22-75), 58(55%) were male. No CML therapy during COVID-19 was in 34 (32%) pts (interrupted or not started), therapy was held in 67 (63%), no data in 5 (5%). Disease course of COVID-19 was asymptomatic, mild, moderate and severe in 5 (5%), 59 (56%), 29(27%) and 13(12%) pts respectively. No therapy for COVID-19 was in 30 (28%) pts, symptomatic therapy (fever relief, anti-cough, decongestants, vitamins etc) - in 73 (69%) pts, antiviral drugs and antibiotics were used in 28 (26%) and 44 (42%) pts respectively. Anticoagulants, corticosteroids and IL-6 inhibitors were introduced mainly during 2nd wave while hydroxychloroquine was abandoned (fig.1). Hospitalization was in 33(31%) pts, oxygenation was applied in 12 (11%) pts. The outcomes were as follows: 101(95%) pts alive (98 recovered, 3 not yet), 4 (3,7%) pts died (2 due to COVID, 2 to CML progression), 1(1,3%) –no data. No COVID reinfection was detected so far. No factors connected with moderate/severe vs asymptomatic/mild COVID-19 were found when analyzing gender, CML phase, TFR, comorbidities, 1st and 2nd wave, except age>52 years (p=0,039). A trend of mild disease was in pts taking TKI vs without TKI (p=0,065).
Seven CP CML pts with Me age 63 years (range 50-70) were vaccinated against COVID-19 since December 2020: 2 had the 1st shot and 5 pts completed vaccination. All 7 pts tolerated the procedure well, with no adverse effects. One pt was checked for antibodies 21 days after the 2nd shot and revealed a high level of anti-SARS-CoV-2 IgG with coefficient of positivity 6,2 (reference range 0-0,9).

Conclusion
The incidence, disease course and COVID-19 dependent mortality rates in CML pts seem to be similar to common population. Older age is a factor of moderate/severe disease course. The TKI impact on COVID-19 course and post-vaccination immunity in CML pts need to be studied.
Keyword(s): Chronic myeloid leukemia, COVID-19, Tyrosine kinase inhibitor


