
Contributions
Abstract: EP670
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Resistance or intolerance to second-generation tyrosine-kinase inhibitors (2GTKIs) is a rough issue in chronic myeloid leukemia (CML) patients. Asciminib, not yet commercially available, inhibits ABL kinase activity by a mechanism distinct to that of currently used TKIs and maintains activity against many resistant forms of BCR-ABL1. Recent data from phase I-III trials showed high response rates with a good safety profile in patients failing to 2GTKIs. However, no studies have specifically addressed response rates with asciminib in ponatinib-pretreated (PPT) patients.
Aims
To present data on responses to asciminib in PPT and non-PPT patients in the setting of clinical practice.
Methods
We gathered retrospective clinical data from 31 patients treated with asciminib after failure of several lines of TKI treatment. Eleven patients received ponatinib at some point for a median time of 18 months. A total of 29 patients were included in the efficacy analysis (2 patients were excluded due to short follow-up). We defined failure as either resistance (BCR-ABL1IS increase despite optimal TKI dosing) or intolerance (unacceptable toxicity leading to TKI termination). Asciminib use was provided by Novartis under a managed-access program (MAP). Data was collected from October 2018 to June 2020 in 25 institutions from the Spanish CML Group (GELMC).
Results
The median time on asciminib for the entire cohort was 9 months (7 in the PPT group and 13 in the non-PPT group). Median time on previous TKI for the entire cohort was 77 months (56 vs 105 for PPT and non-PPT patients, respectively). Median previous TKI lines before asciminib were 4, with no differences for PPT and non-PPT groups. Switch to asciminib was due to resistance in 5/11 (45%) vs 3/20 (15%) for the PPT and non-PPT, respectively, and due to intolerance in 6/11 (55%) and 17/20 (85%) for PPT and non-PPT groups. The median dose of ponatinib was 45 mg in resistant patients and 15 mg in intolerant patients.
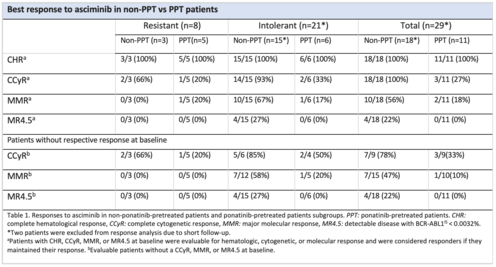
Responses rates according to asciminib indication and previous exposure to ponatinib are shown in table 1. The probabilities of maintaining or improving responses were 100%, 56% and 22% for complete cytogenetic response (CCyR), major molecular response (MMR) and MR4.5 respectively in non-PPT group versus 27%, 18% and 0% in PPT group, respectively. Probability of improving baseline responses were 78%, 47% and 22% for CCyR, MMR and MR4.5 respectively in non-PPT group versus 33%, 10% and 0% respectively observed in PPT patients.
Concerning baseline mutational status, 12 (39%) of the 31 patients displayed BCR-ABL1 mutations (only 1 with T315I), with a heterogeneous pattern and no clear association with outcome: 7 patients in the non-PPT group (35%) and 5 patients in the PPT group (45%).
In terms of tolerability, 10/17 (59%) in the non-PPT-intolerant subgroup displayed side effects; whereas in the PPT-intolerant subgroup 3/6 patients (50%) displayed side effects: grade 1 rash, grade 3 pancreatitis, and grade 4 thrombocytopenia, accordingly.
At the end of follow-up, 27 (87%) continued receiving asciminib, 2 suffered from progression to blast phase and 2 patients showed loss of efficacy; notably, these 4 patients were in the PPT group, whereas all non-PPT continued on asciminib.
Conclusion
Asciminib constitutes a valid treatment option for patients failing 2GTKIs. However, our data suggest that response rates after ponatinib failure are poor, with these patients remaining a high risk group in which alternative treatments are needed.
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor
Abstract: EP670
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Resistance or intolerance to second-generation tyrosine-kinase inhibitors (2GTKIs) is a rough issue in chronic myeloid leukemia (CML) patients. Asciminib, not yet commercially available, inhibits ABL kinase activity by a mechanism distinct to that of currently used TKIs and maintains activity against many resistant forms of BCR-ABL1. Recent data from phase I-III trials showed high response rates with a good safety profile in patients failing to 2GTKIs. However, no studies have specifically addressed response rates with asciminib in ponatinib-pretreated (PPT) patients.
Aims
To present data on responses to asciminib in PPT and non-PPT patients in the setting of clinical practice.
Methods
We gathered retrospective clinical data from 31 patients treated with asciminib after failure of several lines of TKI treatment. Eleven patients received ponatinib at some point for a median time of 18 months. A total of 29 patients were included in the efficacy analysis (2 patients were excluded due to short follow-up). We defined failure as either resistance (BCR-ABL1IS increase despite optimal TKI dosing) or intolerance (unacceptable toxicity leading to TKI termination). Asciminib use was provided by Novartis under a managed-access program (MAP). Data was collected from October 2018 to June 2020 in 25 institutions from the Spanish CML Group (GELMC).
Results
The median time on asciminib for the entire cohort was 9 months (7 in the PPT group and 13 in the non-PPT group). Median time on previous TKI for the entire cohort was 77 months (56 vs 105 for PPT and non-PPT patients, respectively). Median previous TKI lines before asciminib were 4, with no differences for PPT and non-PPT groups. Switch to asciminib was due to resistance in 5/11 (45%) vs 3/20 (15%) for the PPT and non-PPT, respectively, and due to intolerance in 6/11 (55%) and 17/20 (85%) for PPT and non-PPT groups. The median dose of ponatinib was 45 mg in resistant patients and 15 mg in intolerant patients.
Responses rates according to asciminib indication and previous exposure to ponatinib are shown in table 1. The probabilities of maintaining or improving responses were 100%, 56% and 22% for complete cytogenetic response (CCyR), major molecular response (MMR) and MR4.5 respectively in non-PPT group versus 27%, 18% and 0% in PPT group, respectively. Probability of improving baseline responses were 78%, 47% and 22% for CCyR, MMR and MR4.5 respectively in non-PPT group versus 33%, 10% and 0% respectively observed in PPT patients.
Concerning baseline mutational status, 12 (39%) of the 31 patients displayed BCR-ABL1 mutations (only 1 with T315I), with a heterogeneous pattern and no clear association with outcome: 7 patients in the non-PPT group (35%) and 5 patients in the PPT group (45%).
In terms of tolerability, 10/17 (59%) in the non-PPT-intolerant subgroup displayed side effects; whereas in the PPT-intolerant subgroup 3/6 patients (50%) displayed side effects: grade 1 rash, grade 3 pancreatitis, and grade 4 thrombocytopenia, accordingly.
At the end of follow-up, 27 (87%) continued receiving asciminib, 2 suffered from progression to blast phase and 2 patients showed loss of efficacy; notably, these 4 patients were in the PPT group, whereas all non-PPT continued on asciminib.

Conclusion
Asciminib constitutes a valid treatment option for patients failing 2GTKIs. However, our data suggest that response rates after ponatinib failure are poor, with these patients remaining a high risk group in which alternative treatments are needed.
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor


