
Contributions
Abstract: EP667
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Previously reported results from JALSG CML212 (UMIN000007909), a multicentrer open-labeled prospective randomized controlled phase 3 study on de novo chronic myeloid leukemia in chronic phase (CML-CP), showed that cumulative achievement rates of MR4.5 (BCR-ABL1 ≤0.0032% on the International Scale [BCR-ABL1IS]) by 18 months were 33.0% (75/227) (95% CI:27.0–39.6%) in the nilotinib (NIL) arm and 30.8% (70/227) (95% CI: 24.9–37.3%) in the dasatinib arm, with no significant difference.
Aims
JALSG N-STOP216 (UMIN000024984), the next planned study, will evaluate patients with sustained MR4.5, for at least 2 years on frontline NIL for estimating the rate of treatment-free remission (TFR), which is a new treatment goal in CML-CP.
Methods
BCR-ABL-positive CML-CP patients with ≥3 years of frontline NIL and sustained MR4.5 for more than 2 years were enrolled in this trial. Patients restarted NIL after loss of major molecular response (MMR; BCR-ABL1IS ≤0.1%). The data cutoff was December 14, 2020, by which point all patients had completed 12 months of TFR, restarted NIL, or discontinued the study. All patients provided informed consent.
Results
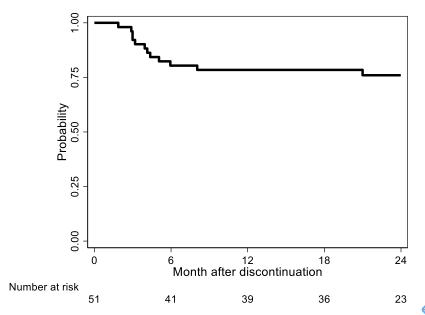
A total of 54 patients were registered from 23 institutes and 51 patients were analyzed as FAS and PPS. Median age of the patients was 53 years (28–79 years). The proportions of Sokal low-, intermediate-, and high-risk groups were 41.2%, 35.3%, and 23.5%, respectively. The proportions of EUTOS low- and high-risk groups were 88.2% and 11.8%, respectively. The median duration of NIL treatment was 53 months (36–129 months), and the median duration of MR4.5 (BCR-ABL1IS ≤0.0032%) was 31 months (24–104 months). The median time to achieved MMR was 183 days (81–1567 days). At 12 months, 39/51 patients remained in TFR (76.5%; 95% CI:62.5%–87.2%) and 12 patients (23.5%) lost MMR. Of 11 patients who restarted NIL, 10 (90.9%) regained MMR in 6 months (1 discontinued study without MMR because of AE). At 12 months, 37/51 patients remained in MR4.0 (BCR-ABL1IS ≤0.01%) (72.6%; 95% CI:58.3%–84.1%); 14 patients (27.5%) lost MR4.0, and 31/51 patients remained in MR4.5 (60.8%; 95% CI:46.1%–74.2%); 20 patients (39.2%) lost MR4.5. No patient experienced disease progression. There was 1 death (pancreas cancer). At 24 months, the treatment-free survival (TFS) rate was 75.9% (95% CI:61.5%–85.6%; Fig.1). As estimated using the Kaplan–Meier method, TFS did not differ among Sokal risk groups (low, int, and high; p=0.5651), EUTOS risk groups (low and high; p=0.5389), duration of NIL (<4 years and ≥ 4 years; p=0.8910), duration of MR4.5 (<30 months and ≥ 30 months; p=0.5852), or time of MMR (<183 days and ≥ 183 days; p=0.1789).
Conclusion
JALSG N-STOP216 provides prospective TFR data in Japanese patients with CML-CP who achieved sustained MR4.5 on frontline NIL. After stopping NIL, 76.5% of patients remained in TFR at 12 months later. The results suggest that a higher proportion of patients with CML-CP who achieved sustained MR4.5 on frontline NIL will remain progression free without treatment compared with that in previous TFR trials.
Keyword(s): Chronic myeloid leukemia, Treatment-free remission, Tyrosine kinase inhibitor
Abstract: EP667
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Clinical
Background
Previously reported results from JALSG CML212 (UMIN000007909), a multicentrer open-labeled prospective randomized controlled phase 3 study on de novo chronic myeloid leukemia in chronic phase (CML-CP), showed that cumulative achievement rates of MR4.5 (BCR-ABL1 ≤0.0032% on the International Scale [BCR-ABL1IS]) by 18 months were 33.0% (75/227) (95% CI:27.0–39.6%) in the nilotinib (NIL) arm and 30.8% (70/227) (95% CI: 24.9–37.3%) in the dasatinib arm, with no significant difference.
Aims
JALSG N-STOP216 (UMIN000024984), the next planned study, will evaluate patients with sustained MR4.5, for at least 2 years on frontline NIL for estimating the rate of treatment-free remission (TFR), which is a new treatment goal in CML-CP.
Methods
BCR-ABL-positive CML-CP patients with ≥3 years of frontline NIL and sustained MR4.5 for more than 2 years were enrolled in this trial. Patients restarted NIL after loss of major molecular response (MMR; BCR-ABL1IS ≤0.1%). The data cutoff was December 14, 2020, by which point all patients had completed 12 months of TFR, restarted NIL, or discontinued the study. All patients provided informed consent.
Results
A total of 54 patients were registered from 23 institutes and 51 patients were analyzed as FAS and PPS. Median age of the patients was 53 years (28–79 years). The proportions of Sokal low-, intermediate-, and high-risk groups were 41.2%, 35.3%, and 23.5%, respectively. The proportions of EUTOS low- and high-risk groups were 88.2% and 11.8%, respectively. The median duration of NIL treatment was 53 months (36–129 months), and the median duration of MR4.5 (BCR-ABL1IS ≤0.0032%) was 31 months (24–104 months). The median time to achieved MMR was 183 days (81–1567 days). At 12 months, 39/51 patients remained in TFR (76.5%; 95% CI:62.5%–87.2%) and 12 patients (23.5%) lost MMR. Of 11 patients who restarted NIL, 10 (90.9%) regained MMR in 6 months (1 discontinued study without MMR because of AE). At 12 months, 37/51 patients remained in MR4.0 (BCR-ABL1IS ≤0.01%) (72.6%; 95% CI:58.3%–84.1%); 14 patients (27.5%) lost MR4.0, and 31/51 patients remained in MR4.5 (60.8%; 95% CI:46.1%–74.2%); 20 patients (39.2%) lost MR4.5. No patient experienced disease progression. There was 1 death (pancreas cancer). At 24 months, the treatment-free survival (TFS) rate was 75.9% (95% CI:61.5%–85.6%; Fig.1). As estimated using the Kaplan–Meier method, TFS did not differ among Sokal risk groups (low, int, and high; p=0.5651), EUTOS risk groups (low and high; p=0.5389), duration of NIL (<4 years and ≥ 4 years; p=0.8910), duration of MR4.5 (<30 months and ≥ 30 months; p=0.5852), or time of MMR (<183 days and ≥ 183 days; p=0.1789).

Conclusion
JALSG N-STOP216 provides prospective TFR data in Japanese patients with CML-CP who achieved sustained MR4.5 on frontline NIL. After stopping NIL, 76.5% of patients remained in TFR at 12 months later. The results suggest that a higher proportion of patients with CML-CP who achieved sustained MR4.5 on frontline NIL will remain progression free without treatment compared with that in previous TFR trials.
Keyword(s): Chronic myeloid leukemia, Treatment-free remission, Tyrosine kinase inhibitor


