
Contributions
Abstract: EP663
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Biology & Translational Research
Background
Long-term survival of patients with chronic myeloid leukemia (CML) has significantly improved since the introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs). Several clinical trials show that approximately half of patients who achieve a sustained deep molecular response during TKI treatment maintain molecular remission after stopping TKIs. There is currently no biomarker that reliably predicts treatment free remission (TFR) in CML, but growing evidence remarks the importance of the immune system, especially of Natural Killer (NK) cells in this scenario.
Aims
Our aim is to discover a biomarker for TFR prediction by analyzing the conventional and adaptive phenotype of NK cells at the time of discontinuation and their relation to successful TKI cessation, and to assess any phenotypic changes throughout the months without treatment.
Methods
Fifty chronic phase CML patients who participated in the Argentina Stop Trial were recruited from 7 Argentinian centers. Peripheral blood samples were collected before TKI stopping and then at months 3, 12 and at any time when MR3.0 was lost. Freshly isolated mononuclear cells were immunophenotyped by staining with CD3, CD16, CD25, CD56, CD57, CD158, NKp30, NKp44, NKp46, NKG2A, NKG2C, NKG2D and PD-1 antibodies and subpopulations of NK cells were analyzed by flow cytometry (BD FACS Canto™II). Molecular recurrence-free survival was estimated by the Kaplan–Meier method. Mann Whitney test, Wilcoxon test or paired T test were performed to compare variables between groups, where p<0.05 was considered statistically significant. Quantitative variables were dichotomized according to ROC curves. Survival curves were compared with log rank test.
Results
Forty-six patients were analyzed with a median follow-up of 15 months (range 12-21) and with an estimated molecular recurrence-free survival of 67.4% at 12 months.
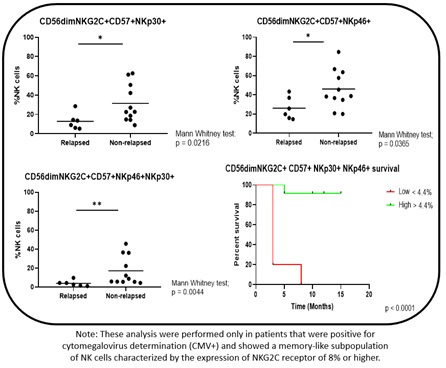
At the time of discontinuation, non-relapsing patients had significantly higher frequencies of NK subpopulations with cytomegalovirus-related memory features (CD56dimCD57+NKG2C+), but higher expression of cytotoxicity markers NKp30high (p=0.0216) or NKp46high (p=0.0365). Most important, a higher coexpression of NKp30 and NKp46 on CD56dimCD57+NKG2C+ subpopulation (defined as memory-like NK, ML-NK) showed a significant difference in survival when relapsed vs non-relapsed patients were compared (0% vs 92% at 12 months, log rank test, p<0.0001) (Fig. 1). After TKI discontinuation, a significant and sustained increase or decrease over time could be seen for 4 NK cells subpopulations expressing CD57, NKG2D, PD-1 and NKp44, while NKp30, CD158, CD57+CD16+CD158+, and ML-NK subpopulations did not significantly change over the course of 12 months.
Conclusion
Our study is the first, to our knowledge, to describe this adaptive-like subpopulations of NK cells with enhanced cytotoxicity-related phenotype which could have a protecting role in patients who do not relapse. Moreover, the ML-NK phenotype was retained more efficiently in TFR patients off-therapy, suggesting that the restored immune control observed at the time of discontinuation is not an entirely TKI-mediated effect, which could be used as a predictive biomarker. The clinical impact of NK cells in patients who have discontinued TKIs is still controversial. To fully understand their role, we are planning to carry out functional studies
Keyword(s): Chronic myeloid leukemia, Flow cytometry, Immunophenotype, Natural killer
Abstract: EP663
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Biology & Translational Research
Background
Long-term survival of patients with chronic myeloid leukemia (CML) has significantly improved since the introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs). Several clinical trials show that approximately half of patients who achieve a sustained deep molecular response during TKI treatment maintain molecular remission after stopping TKIs. There is currently no biomarker that reliably predicts treatment free remission (TFR) in CML, but growing evidence remarks the importance of the immune system, especially of Natural Killer (NK) cells in this scenario.
Aims
Our aim is to discover a biomarker for TFR prediction by analyzing the conventional and adaptive phenotype of NK cells at the time of discontinuation and their relation to successful TKI cessation, and to assess any phenotypic changes throughout the months without treatment.
Methods
Fifty chronic phase CML patients who participated in the Argentina Stop Trial were recruited from 7 Argentinian centers. Peripheral blood samples were collected before TKI stopping and then at months 3, 12 and at any time when MR3.0 was lost. Freshly isolated mononuclear cells were immunophenotyped by staining with CD3, CD16, CD25, CD56, CD57, CD158, NKp30, NKp44, NKp46, NKG2A, NKG2C, NKG2D and PD-1 antibodies and subpopulations of NK cells were analyzed by flow cytometry (BD FACS Canto™II). Molecular recurrence-free survival was estimated by the Kaplan–Meier method. Mann Whitney test, Wilcoxon test or paired T test were performed to compare variables between groups, where p<0.05 was considered statistically significant. Quantitative variables were dichotomized according to ROC curves. Survival curves were compared with log rank test.
Results
Forty-six patients were analyzed with a median follow-up of 15 months (range 12-21) and with an estimated molecular recurrence-free survival of 67.4% at 12 months.
At the time of discontinuation, non-relapsing patients had significantly higher frequencies of NK subpopulations with cytomegalovirus-related memory features (CD56dimCD57+NKG2C+), but higher expression of cytotoxicity markers NKp30high (p=0.0216) or NKp46high (p=0.0365). Most important, a higher coexpression of NKp30 and NKp46 on CD56dimCD57+NKG2C+ subpopulation (defined as memory-like NK, ML-NK) showed a significant difference in survival when relapsed vs non-relapsed patients were compared (0% vs 92% at 12 months, log rank test, p<0.0001) (Fig. 1). After TKI discontinuation, a significant and sustained increase or decrease over time could be seen for 4 NK cells subpopulations expressing CD57, NKG2D, PD-1 and NKp44, while NKp30, CD158, CD57+CD16+CD158+, and ML-NK subpopulations did not significantly change over the course of 12 months.

Conclusion
Our study is the first, to our knowledge, to describe this adaptive-like subpopulations of NK cells with enhanced cytotoxicity-related phenotype which could have a protecting role in patients who do not relapse. Moreover, the ML-NK phenotype was retained more efficiently in TFR patients off-therapy, suggesting that the restored immune control observed at the time of discontinuation is not an entirely TKI-mediated effect, which could be used as a predictive biomarker. The clinical impact of NK cells in patients who have discontinued TKIs is still controversial. To fully understand their role, we are planning to carry out functional studies
Keyword(s): Chronic myeloid leukemia, Flow cytometry, Immunophenotype, Natural killer


