
Contributions
Abstract: EP658
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Biology & Translational Research
Background
Chronic Myeloid Leukemia (CML) is a myeloproliferative disease caused by the 9;22 chromosomal translocation which leads to the formation of the chimeric kinase BCR-ABL. The introduction of imatinib and other tyrosine kinase inhibitors (TKI) allowed excellent control of the disease in the majority of patients. In a number of patients, after a prolonged treatment, Ph+ cells become undetectable or below 10-4, thus allowing a TKI discontinuation attempt. However, about half of CML patients experience a molecular relapse, which in 80% of cases occurs within 6 months from interruption, and need to resume treatment. There is currently no marker to predict the outcome of discontinuation.
Aims
Previous studies have shown that age can predict relapse after TKI discontinuation. We hypothesized that this may be linked to the DDR phenotype of leukemic stem cells (LSCs). We aimed to analyze the DDR status of LSCs, measured at diagnosis, in CML patients who attempted TKI discontinuation, and correlated it with treatment-free remission (TFR) duration.
Methods
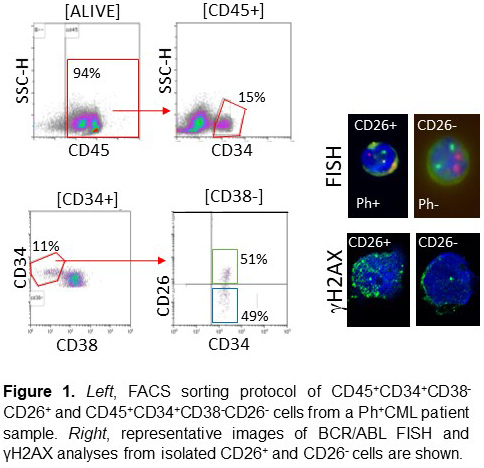
CD26 has been proposed as a marker of CML LSCs. We sorted LSCs (CD45+CD34+CD38-CD26+) and normal HSCs (CD45+CD34+CD38-CD26-) from bone marrow samples obtained at diagnosis in 27 CP-CML patients who later discontinued TKI (imatinib, n=15; other TKIs, n=12). The analysis of DDR phenotype was performed by γH2AX immunofluorescence at confocal microscope. Clinical and molecular data were correlated with discontinuation outcome.
Results
CD26 staining correctly identified Ph+ cells within CD34+CD38- cells (Figure 1). We found that DDR in the CD34+CD38- population correlates with patients’ age (p = 0.05) and that CD26+ LSCs present greater DDR compared to normal CD26- stem cells (117±48.9 vs 48.8±19.9; p<0.0001, Figure 1). A correlation was also found between DDR at diagnoses and the outcome of TKI discontinuation, which identifies patients in durable TFR as those with less DNA damage at onset (72.2±32.4 vs 107.6±18; p = 0.034). Another interesting finding of this study was the duration of molecular remission before the discontinuation: in fact, it was clear that the longer the duration of deep molecular remission, the lower the probability of relapse after the TKI discontinuation (p = 0.026).
Conclusion
We identified DDR and duration of remission as potential biomarkers of relapse in CML patients after TKI discontinuation. Our data also indicate that CML LSCs possess a more pronounced DDR phenotype compared to normal HSCs. These results need to be validated in a larger cohort of patients.
Keyword(s): Chronic myeloid leukemia, DNA damage, Treatment-free remission
Abstract: EP658
Type: E-Poster Presentation
Session title: Chronic myeloid leukemia - Biology & Translational Research
Background
Chronic Myeloid Leukemia (CML) is a myeloproliferative disease caused by the 9;22 chromosomal translocation which leads to the formation of the chimeric kinase BCR-ABL. The introduction of imatinib and other tyrosine kinase inhibitors (TKI) allowed excellent control of the disease in the majority of patients. In a number of patients, after a prolonged treatment, Ph+ cells become undetectable or below 10-4, thus allowing a TKI discontinuation attempt. However, about half of CML patients experience a molecular relapse, which in 80% of cases occurs within 6 months from interruption, and need to resume treatment. There is currently no marker to predict the outcome of discontinuation.
Aims
Previous studies have shown that age can predict relapse after TKI discontinuation. We hypothesized that this may be linked to the DDR phenotype of leukemic stem cells (LSCs). We aimed to analyze the DDR status of LSCs, measured at diagnosis, in CML patients who attempted TKI discontinuation, and correlated it with treatment-free remission (TFR) duration.
Methods
CD26 has been proposed as a marker of CML LSCs. We sorted LSCs (CD45+CD34+CD38-CD26+) and normal HSCs (CD45+CD34+CD38-CD26-) from bone marrow samples obtained at diagnosis in 27 CP-CML patients who later discontinued TKI (imatinib, n=15; other TKIs, n=12). The analysis of DDR phenotype was performed by γH2AX immunofluorescence at confocal microscope. Clinical and molecular data were correlated with discontinuation outcome.
Results
CD26 staining correctly identified Ph+ cells within CD34+CD38- cells (Figure 1). We found that DDR in the CD34+CD38- population correlates with patients’ age (p = 0.05) and that CD26+ LSCs present greater DDR compared to normal CD26- stem cells (117±48.9 vs 48.8±19.9; p<0.0001, Figure 1). A correlation was also found between DDR at diagnoses and the outcome of TKI discontinuation, which identifies patients in durable TFR as those with less DNA damage at onset (72.2±32.4 vs 107.6±18; p = 0.034). Another interesting finding of this study was the duration of molecular remission before the discontinuation: in fact, it was clear that the longer the duration of deep molecular remission, the lower the probability of relapse after the TKI discontinuation (p = 0.026).

Conclusion
We identified DDR and duration of remission as potential biomarkers of relapse in CML patients after TKI discontinuation. Our data also indicate that CML LSCs possess a more pronounced DDR phenotype compared to normal HSCs. These results need to be validated in a larger cohort of patients.
Keyword(s): Chronic myeloid leukemia, DNA damage, Treatment-free remission


