
Contributions
Abstract: EP654
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
The Belgian ibrutinib Real-World (RW) Data (BiRD) study is a multicenter, observational study of adults with a confirmed diagnosis of CLL, MCL, or Waldenström’s macroglobulinemia (WM) eligible for reimbursed ibrutinib treatment at treatment initiation or participating in a Medical Need Program and switched to reimbursed treatment in Belgium. RW studies provide valuable insights into ibrutinib effectiveness and safety in routine clinical practice. Here we present updated results from the third interim analyses (IA) with a median follow-up of 34.3 months for the CLL cohort and 35.8 months for the MCL cohort.
Aims
To assess ibrutinib outcomes (primary: progression-free survival [PFS] and overall response rate [ORR]; secondary: included overall survival [OS] and safety) in RW patients (pts) with previously untreated or relapsed or refractory (R/R) CLL and R/R MCL in Belgium.
Methods
Data were collected both prospectively (pro) and retrospectively (ret). Total population results were adjusted for left truncation.
Results
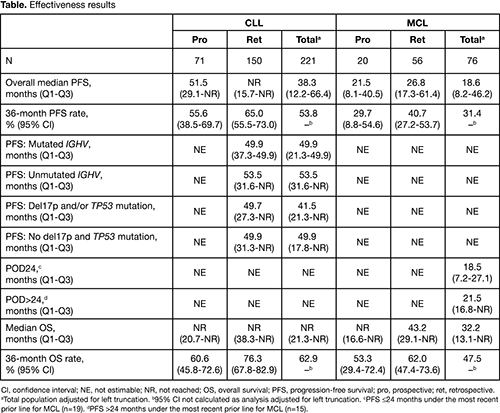
Effectiveness results are presented in the Table.
In the CLL cohort, 221 pts (pro n=71; ret n=150) were included in the effectiveness population; 64.3% were male, median age at ibrutinib initiation was 72 (range, 38-90) years, median time from diagnosis to ibrutinib initiation was 5.8 years, 27.4% were previously untreated 59.1%, had del17p and/or TP53 mutation, and 72.5% had no IGHV mutation. The median PFS for CLL was 38.3 months (51.5/not reached [NR] months in the pro/ret cohorts). ORR was 90.0% (complete response [CR] 16.7%, partial response [PR] 51.6%, PR with lymphocytosis 21.7%). Median OS was NR. Of the 226 pts in the CLL safety population, 96.9% had ≥1 treatment-emergent adverse event (TEAE) and 52.2% had ≥1 serious TEAE. TEAEs led to dose reduction, interruption, or discontinuation in 21.2%, 31.9%, and 26.5% of pts, respectively. TEAEs of interest in pts with CLL reported as % (% difference from second IA, after an additional 15.3 months of follow-up) were: major bleeding 4.9% (-0.4%); infection 66.4% (9.3%); hypertension 17.3% (5.1%); atrial fibrillation (AF) 10.6% (3.2%); arthralgia/myalgia 16.8% (4.1%); diarrhea 21.7% (3.2%). Median treatment duration for pts with CLL was 25.5 months (19.6/28.6 months pro/ret), and 135/226 pts (58.7%) remain on treatment.
In the MCL cohort, 76 pts (pro n=20; ret n=56) were included in the effectiveness population; 76.3% were male, median age at ibrutinib initiation was 74 (range, 47-88) years, and median time from diagnosis to ibrutinib initiation was 2.8 years. The median PFS for MCL was 18.6 months (21.5/26.8 months in the pro/ret cohorts). ORR was 93.4% (CR 39.5%, PR 53.9%). Median OS was 32.2 months. Of the 76 pts in the MCL safety population, all had ≥1 TEAE and 67.1% had ≥1 serious TEAE. TEAEs led to dose reduction, interruption, or discontinuation in 31.6%, 43.4%, and 26.3% of pts, respectively. TEAEs of interest in pts with MCL reported as % (% difference from second IA, after an additional 15.2 months of follow-up) were: major bleeding 7.9% (1.3%); infection 64.5% (6.6%); hypertension 6.6% (0%); AF 21.1% (4.0%); arthralgia/myalgia 11.8% (0%); diarrhea 35.5% (5.2%). Median treatment duration for pts with MCL was 24.7 months (22.8/25.3 months pro/ret), and 26/76 pts (34.2%) remain on treatment.
Conclusion
Results over a median follow-up of 35+ months from the BiRD study confirm the sustained effectiveness of ibrutinib in both CLL and MCL. TEAEs were manageable in elderly patients with no new safety signals.
Keyword(s): Chronic lymphocytic leukemia, Ibrutinib, Mantle cell lymphoma
Abstract: EP654
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
The Belgian ibrutinib Real-World (RW) Data (BiRD) study is a multicenter, observational study of adults with a confirmed diagnosis of CLL, MCL, or Waldenström’s macroglobulinemia (WM) eligible for reimbursed ibrutinib treatment at treatment initiation or participating in a Medical Need Program and switched to reimbursed treatment in Belgium. RW studies provide valuable insights into ibrutinib effectiveness and safety in routine clinical practice. Here we present updated results from the third interim analyses (IA) with a median follow-up of 34.3 months for the CLL cohort and 35.8 months for the MCL cohort.
Aims
To assess ibrutinib outcomes (primary: progression-free survival [PFS] and overall response rate [ORR]; secondary: included overall survival [OS] and safety) in RW patients (pts) with previously untreated or relapsed or refractory (R/R) CLL and R/R MCL in Belgium.
Methods
Data were collected both prospectively (pro) and retrospectively (ret). Total population results were adjusted for left truncation.
Results
Effectiveness results are presented in the Table.
In the CLL cohort, 221 pts (pro n=71; ret n=150) were included in the effectiveness population; 64.3% were male, median age at ibrutinib initiation was 72 (range, 38-90) years, median time from diagnosis to ibrutinib initiation was 5.8 years, 27.4% were previously untreated 59.1%, had del17p and/or TP53 mutation, and 72.5% had no IGHV mutation. The median PFS for CLL was 38.3 months (51.5/not reached [NR] months in the pro/ret cohorts). ORR was 90.0% (complete response [CR] 16.7%, partial response [PR] 51.6%, PR with lymphocytosis 21.7%). Median OS was NR. Of the 226 pts in the CLL safety population, 96.9% had ≥1 treatment-emergent adverse event (TEAE) and 52.2% had ≥1 serious TEAE. TEAEs led to dose reduction, interruption, or discontinuation in 21.2%, 31.9%, and 26.5% of pts, respectively. TEAEs of interest in pts with CLL reported as % (% difference from second IA, after an additional 15.3 months of follow-up) were: major bleeding 4.9% (-0.4%); infection 66.4% (9.3%); hypertension 17.3% (5.1%); atrial fibrillation (AF) 10.6% (3.2%); arthralgia/myalgia 16.8% (4.1%); diarrhea 21.7% (3.2%). Median treatment duration for pts with CLL was 25.5 months (19.6/28.6 months pro/ret), and 135/226 pts (58.7%) remain on treatment.
In the MCL cohort, 76 pts (pro n=20; ret n=56) were included in the effectiveness population; 76.3% were male, median age at ibrutinib initiation was 74 (range, 47-88) years, and median time from diagnosis to ibrutinib initiation was 2.8 years. The median PFS for MCL was 18.6 months (21.5/26.8 months in the pro/ret cohorts). ORR was 93.4% (CR 39.5%, PR 53.9%). Median OS was 32.2 months. Of the 76 pts in the MCL safety population, all had ≥1 TEAE and 67.1% had ≥1 serious TEAE. TEAEs led to dose reduction, interruption, or discontinuation in 31.6%, 43.4%, and 26.3% of pts, respectively. TEAEs of interest in pts with MCL reported as % (% difference from second IA, after an additional 15.2 months of follow-up) were: major bleeding 7.9% (1.3%); infection 64.5% (6.6%); hypertension 6.6% (0%); AF 21.1% (4.0%); arthralgia/myalgia 11.8% (0%); diarrhea 35.5% (5.2%). Median treatment duration for pts with MCL was 24.7 months (22.8/25.3 months pro/ret), and 26/76 pts (34.2%) remain on treatment.

Conclusion
Results over a median follow-up of 35+ months from the BiRD study confirm the sustained effectiveness of ibrutinib in both CLL and MCL. TEAEs were manageable in elderly patients with no new safety signals.
Keyword(s): Chronic lymphocytic leukemia, Ibrutinib, Mantle cell lymphoma


