
Contributions
Abstract: EP650
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Diffuse large B-cell (DLBCL) transformation from chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), Richter Syndrome (RS), has poor prognosis. Venetoclax, Bcl-2 inhibitor, has shown activity in DLBCL-RS. Atezolizumab is a humanized monoclonal antibody (MoAb) targeting PD-L1 on tumor-infiltrating immune cells/tumor cells and prevents PD-1 receptor-B7.1 interaction. Obinutuzumab, antiCD-20 MoAb, presents greater antibody-dependent (AD) cellular cytotoxicity, AD cellular phagocytosis and direct cell death compared to rituximab. Preliminary data showed safety and activity of atezolizumab+/-obinutuzumab in heavily pretreated DLBCL.
Aims
MOLTO is a multicenter international phase II study (EUDRACT 2018-005028-40) evaluating safety and efficacy of venetoclax, atezolizumab, obinutuzumab combination in 28 RS.
Methods
Treatment consists of 35 cycles with obinutuzumab (1000 mg C1-8), atezolizumab (1200 mg C1-18) and venetoclax (400 mg/d C1-35), in untreated DLBCL-RS from CLL/SLL. Initial safety run phase consists of 9 pts receiving at least 3 courses. Enrollment would have been stopped in case of ≥3 non-infective/non-hematologic therapy-related G≥4 adverse events (AEs). Remaining pts received same treatment schedule of initial safety cohort.
Results
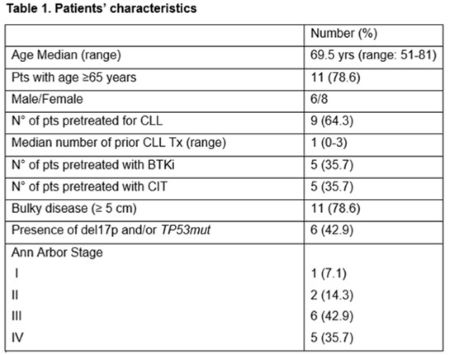
Enrollment started from October 2019. As none of the 9 pts of the safety run phase developed an infective and/or non-hematological G ≥4 AE, enrollment went on and, as of February 2021, a total of 14 pts received at least 1 cycle of therapy. In table 1 are shown pts characteristics. Overall, 110 cycles have been administered. Five serious AE (SAEs) were recorded in 4 pts: 1 fever of unknown origin (FUO) and 1 case of autoimmune encephalitis, both G3, resolved and not leading to treatment discontinuation, and 1 G5 pneumonia. The remaining 2 SAEs were related to hospitalization due to disease progression (PD). Twelve/14 pts (85.7%) developed at least 1 AE of any grade. Severe (G 3-5) hematological toxicity was recorded in 7 pts, while 5 pts (including 3 developing SAEs) experienced severe non-hematological toxicity. The most common hematological toxicity was thrombocytopenia: 20 episodes/110 courses (18.2%), 9 being G≥3. Other G≥3 hematologic toxicities included: 10% neutropenia and 0.9% anemia. Two severe non-hematological toxicities were recorded: 1 hyperamylasemia and 1 hypercalcemia. Seven pts (50%) experienced a grade 2 FUO and only 1 major infection was observed. Two were the AE of special interest: 1 autoimmune myositis and 1 autoimmune encephalitis both successfully managed with steroids. No G≥3 infusion related reactions (IRR) occurred and all G1/2 have been considered associated to obinutuzumab. Venetoclax ramp-up was successfully completed in all pts, in 2 cases accelerated dose-escalation (Koening et al. 2020) was performed due to rapidly PD. No laboratory or clinical tumor lysis syndrome (TLS) was recorded. Overall, 9 pts are still on treatment with a median time on study of 4.4 (range 1 - 16.7) m, only 1/5 pts discontinued treatment due to toxicity.
Conclusion
The combination venetoclax, obinutuzumab and atezolizumab was well tolerated even in elderly pts. In the absence of significative warning, Independent Data Monitoring Committee allowed the accomplishment of safety run phase. Obinutuzumab-atezolizumab combination did not result in an enhanced rate or severity of IRR. In this highly proliferating disease, venetoclax did not lead to TLS even when applying accelerated ramp-up. Accrual is ongoing, and updated results will be presented at the meeting.
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Immunotherapy, Transformation
Abstract: EP650
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Diffuse large B-cell (DLBCL) transformation from chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), Richter Syndrome (RS), has poor prognosis. Venetoclax, Bcl-2 inhibitor, has shown activity in DLBCL-RS. Atezolizumab is a humanized monoclonal antibody (MoAb) targeting PD-L1 on tumor-infiltrating immune cells/tumor cells and prevents PD-1 receptor-B7.1 interaction. Obinutuzumab, antiCD-20 MoAb, presents greater antibody-dependent (AD) cellular cytotoxicity, AD cellular phagocytosis and direct cell death compared to rituximab. Preliminary data showed safety and activity of atezolizumab+/-obinutuzumab in heavily pretreated DLBCL.
Aims
MOLTO is a multicenter international phase II study (EUDRACT 2018-005028-40) evaluating safety and efficacy of venetoclax, atezolizumab, obinutuzumab combination in 28 RS.
Methods
Treatment consists of 35 cycles with obinutuzumab (1000 mg C1-8), atezolizumab (1200 mg C1-18) and venetoclax (400 mg/d C1-35), in untreated DLBCL-RS from CLL/SLL. Initial safety run phase consists of 9 pts receiving at least 3 courses. Enrollment would have been stopped in case of ≥3 non-infective/non-hematologic therapy-related G≥4 adverse events (AEs). Remaining pts received same treatment schedule of initial safety cohort.
Results
Enrollment started from October 2019. As none of the 9 pts of the safety run phase developed an infective and/or non-hematological G ≥4 AE, enrollment went on and, as of February 2021, a total of 14 pts received at least 1 cycle of therapy. In table 1 are shown pts characteristics. Overall, 110 cycles have been administered. Five serious AE (SAEs) were recorded in 4 pts: 1 fever of unknown origin (FUO) and 1 case of autoimmune encephalitis, both G3, resolved and not leading to treatment discontinuation, and 1 G5 pneumonia. The remaining 2 SAEs were related to hospitalization due to disease progression (PD). Twelve/14 pts (85.7%) developed at least 1 AE of any grade. Severe (G 3-5) hematological toxicity was recorded in 7 pts, while 5 pts (including 3 developing SAEs) experienced severe non-hematological toxicity. The most common hematological toxicity was thrombocytopenia: 20 episodes/110 courses (18.2%), 9 being G≥3. Other G≥3 hematologic toxicities included: 10% neutropenia and 0.9% anemia. Two severe non-hematological toxicities were recorded: 1 hyperamylasemia and 1 hypercalcemia. Seven pts (50%) experienced a grade 2 FUO and only 1 major infection was observed. Two were the AE of special interest: 1 autoimmune myositis and 1 autoimmune encephalitis both successfully managed with steroids. No G≥3 infusion related reactions (IRR) occurred and all G1/2 have been considered associated to obinutuzumab. Venetoclax ramp-up was successfully completed in all pts, in 2 cases accelerated dose-escalation (Koening et al. 2020) was performed due to rapidly PD. No laboratory or clinical tumor lysis syndrome (TLS) was recorded. Overall, 9 pts are still on treatment with a median time on study of 4.4 (range 1 - 16.7) m, only 1/5 pts discontinued treatment due to toxicity.

Conclusion
The combination venetoclax, obinutuzumab and atezolizumab was well tolerated even in elderly pts. In the absence of significative warning, Independent Data Monitoring Committee allowed the accomplishment of safety run phase. Obinutuzumab-atezolizumab combination did not result in an enhanced rate or severity of IRR. In this highly proliferating disease, venetoclax did not lead to TLS even when applying accelerated ramp-up. Accrual is ongoing, and updated results will be presented at the meeting.
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Immunotherapy, Transformation


