
Contributions
Abstract: EP649
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Recommendations for venetoclax initiation in Chronic Lymphocytic Leukaemia (CLL) include inpatient admission during ramp-up dosing for patients at High-risk of Tumour Lysis Syndrome(TLS). Outpatient-based monitoring for high-risk TLS risk subgroup has been implemented in some centres. It is of interest to evaluate patterns of TLS monitoring and safety of this practice in the real world setting.
Aims
The primary objective is to evaluate TLS incidence CLL patients treated with venetoclax outpatient ramp-up in routine clinical practice. Secondary objectives are identifying the dose and time-point in which TLS occurs, management of TLS, TLS outcomes and impact on treatment delivery.
Methods
We performed aUK multi-centre, retrospective observational study, analysing consecutive adult CLL patients treated with venetoclax monotherapy or in combination with anti-CD20 monoclonal antibody (May/2016 to December/2020). Patients treated within clinical trials were excluded. Key outcomes were: Development of biochemical or clinical TLS after each venetoclax dose escalation, frequency of TLS between outpatient and inpatient ramp-up strategy, treatment delay/discontinuation after TLS events, frequency of rasburicase use for TLS treatment and TLS-related morbidity and mortality. Response rate using iwCLL criteria was collected.
Results
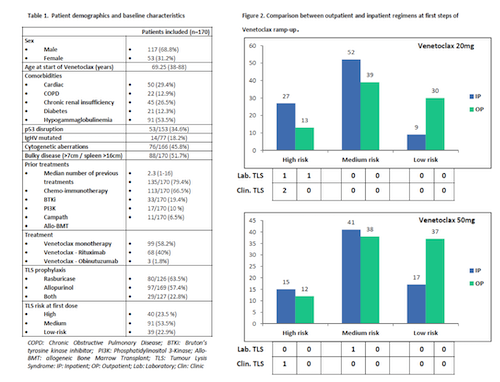
170 patients from 11 UK centres were included. Patient’s characteristics are shown in Table 1. 17p deletion and/or TP53 mutation was present in 34.5% (n=53), 51.7% had bulky disease (>7cm). 66.5% were previously Bruton Tyrosine Kinase inhibitor (BTKi) exposed. 99 patients (58.2%) had venetoclax monotherapy. All patients received TLS prophylaxis during the first steps of escalation, 63.2% with rasburicase. 23.5% (n=40) of patients had high TLS risk at initial ramp up. 127 high-TLS-risk dose escalations occurred, 52% (N=66) as inpatient. Overall, 7 TLS events were observed (3 clinical, 4 biochemical), 6/7 in the high-risk group (4.7% of high-risk escalations). 2/7 were outpatients at TLS onset and were admitted, 4/7 received emergency rasburicase and 1/7 required haemodialysis. There were no deaths or treatment discontinuations related to TLS, 6/7 patients had the next dose delayed. Most TLS events occurred at 20 and 50mg dose level and at the 6h post-dose timepoint (Fig. 2). 46 episodes of single laboratory abnormality were observed. Raised serum phosphate the most frequent (67.4%), 15.2% required admission and 17.4% patients had subsequent dose delayed for that reason. Complete and partial response rates were 45.9% and 39.1% respectively. All patients who experienced TLS episodes achieved some grade of response (CR or PR). CR rate in patients with isolated hyperphosphatemia was 80%.
Conclusion
Our real-word observational study revealed thathigh-risk patients could be managed as outpatients without a significant increase in TLS events or admission compared to impatient escalation. As previously described, TLS occurred predominantly at initial ramp up stages and early timepoints. We observed higher than expected proportion of inpatient ramp up medium and low risk patients. Despite low numbers, no deaths or discontinuations due to TLS were observed. Single laboratory abnormalities were frequently observed, mainly hyperphosphatemia, but only rarely translated into dose delays or TLS. Higher CR rates were positively associated with TLS and isolated hyperphosphatemia.
Keyword(s): Chronic lymphocytic leukemia, Tumor lysis
Abstract: EP649
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Recommendations for venetoclax initiation in Chronic Lymphocytic Leukaemia (CLL) include inpatient admission during ramp-up dosing for patients at High-risk of Tumour Lysis Syndrome(TLS). Outpatient-based monitoring for high-risk TLS risk subgroup has been implemented in some centres. It is of interest to evaluate patterns of TLS monitoring and safety of this practice in the real world setting.
Aims
The primary objective is to evaluate TLS incidence CLL patients treated with venetoclax outpatient ramp-up in routine clinical practice. Secondary objectives are identifying the dose and time-point in which TLS occurs, management of TLS, TLS outcomes and impact on treatment delivery.
Methods
We performed aUK multi-centre, retrospective observational study, analysing consecutive adult CLL patients treated with venetoclax monotherapy or in combination with anti-CD20 monoclonal antibody (May/2016 to December/2020). Patients treated within clinical trials were excluded. Key outcomes were: Development of biochemical or clinical TLS after each venetoclax dose escalation, frequency of TLS between outpatient and inpatient ramp-up strategy, treatment delay/discontinuation after TLS events, frequency of rasburicase use for TLS treatment and TLS-related morbidity and mortality. Response rate using iwCLL criteria was collected.
Results
170 patients from 11 UK centres were included. Patient’s characteristics are shown in Table 1. 17p deletion and/or TP53 mutation was present in 34.5% (n=53), 51.7% had bulky disease (>7cm). 66.5% were previously Bruton Tyrosine Kinase inhibitor (BTKi) exposed. 99 patients (58.2%) had venetoclax monotherapy. All patients received TLS prophylaxis during the first steps of escalation, 63.2% with rasburicase. 23.5% (n=40) of patients had high TLS risk at initial ramp up. 127 high-TLS-risk dose escalations occurred, 52% (N=66) as inpatient. Overall, 7 TLS events were observed (3 clinical, 4 biochemical), 6/7 in the high-risk group (4.7% of high-risk escalations). 2/7 were outpatients at TLS onset and were admitted, 4/7 received emergency rasburicase and 1/7 required haemodialysis. There were no deaths or treatment discontinuations related to TLS, 6/7 patients had the next dose delayed. Most TLS events occurred at 20 and 50mg dose level and at the 6h post-dose timepoint (Fig. 2). 46 episodes of single laboratory abnormality were observed. Raised serum phosphate the most frequent (67.4%), 15.2% required admission and 17.4% patients had subsequent dose delayed for that reason. Complete and partial response rates were 45.9% and 39.1% respectively. All patients who experienced TLS episodes achieved some grade of response (CR or PR). CR rate in patients with isolated hyperphosphatemia was 80%.

Conclusion
Our real-word observational study revealed thathigh-risk patients could be managed as outpatients without a significant increase in TLS events or admission compared to impatient escalation. As previously described, TLS occurred predominantly at initial ramp up stages and early timepoints. We observed higher than expected proportion of inpatient ramp up medium and low risk patients. Despite low numbers, no deaths or discontinuations due to TLS were observed. Single laboratory abnormalities were frequently observed, mainly hyperphosphatemia, but only rarely translated into dose delays or TLS. Higher CR rates were positively associated with TLS and isolated hyperphosphatemia.
Keyword(s): Chronic lymphocytic leukemia, Tumor lysis


