
Contributions
Abstract: EP644
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
ADMIRE and ARCTIC were phase II randomised controlled trials recruiting between July 2009-September 2012 comparing fludarabine, cyclophosphamide and rituximab (FCR) with or without mitoxantrone (FCMR) or the addition of mitoxantrone with low dose of rituximab (FCM-miniR).
Aims
To assess how pre-specified prognostic factors, minimal residual disease (MRD), toxicity and number of treatment cycles affect progression free survival (PFS) and overall survival (OS) in the combined ADMIRE/ARCTIC cohort.
Methods
ARCTIC/ADMIRE provided long-term data on 415 previously untreated CLL patients. Kaplan-Meier survival estimates, and Cox proportional hazards models assessed PFS and OS according to potential prognostic factors, toxicities and treatment cycles received. Penalised multivariable Cox regression identified the prognostic factors predictive of PFS and OS. Multiple imputation by chained equations was used to account for missing data.
Results
Median duration of follow-up is 84 months. There is no significant difference in the hazard ratios for PFS or OS between FCR and FCM-R and between FCR and FCM-miniR. Overall PFS is 65 months (95% confidence interval (CI) 56,72) and OS is 108 months (CI 101,not reached).
Univariable Cox regression analysis of PFS by pre-treatment prognostic factors revealed unmutated IGHV genes (UM-IGHV), 17p/11q deletion, TP53 mutations, CLL-IPI, %CD38+ cells, decreasing CD20 mean fluorescence intensity (MFI), %CD49d+ cells, CCR6 (MFI and %+ cells) and LAIR1 MFI to be associated with shortened PFS. A baseline multivariable penalised Cox regression analysis of PFS found UM-IGHV, CCR6, 17p/11q deletion, TP53 mutations, CD49d and SF3B1 mutations remained prognostic for PFS.
Similar univariable analysis of OS found increasing age, positive direct Coombs test, UM-IGHV, deletion 17p, TP53 mutations, CLL-IPI and %CD49d+ cells, and decreasing CCR6 MFI and LAIR1 MFI to be associated with shortened OS. Multivariable penalised Cox regression by pre-treatment factors found age, %CD49d+ cells, d17p, TP53 mutations and direct Coombs test remained prognostic for OS. Mutations in ATM, BIRC3, NOTCH1 and SF3B1 are not associated with shortened OS.
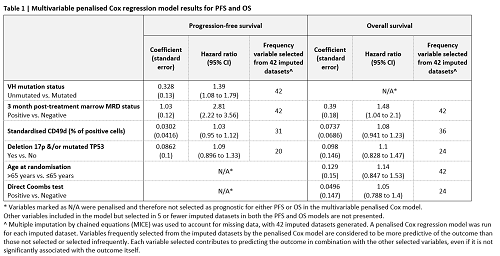
Detectable marrow MRD (>10-4) 3 months after therapy completion is associated with inferior PFS and OS on univariable analysis. Incorporation of MRD into a prognostic multivariable model revealed that UM-IGHV, MRD, d17p/TP53 mutations and %CD49d+ cells are predictive of PFS. In the same model for OS age, MRD, %CD49d+ cells, d17p/TP53 mutations and direct coombs test are predictive of OS (Table 1).
Grade 3/4 infection-related toxicities are associated with inferior PFS and OS however the occurrence of other toxicities are not associated with inferior outcomes. Hypogammaglobulinemia is not associated with the probability of grade 3/4 infection or shortened PFS/OS. Participants who received 3 cycles or less of the prescribed 6 cycles for reasons other than progressive disease had inferior PFS and OS and more likely to have other poor prognostic features.
Conclusion
A multivariable analysis of prognostic factors and MRD reveals the key prognostic factors for shortened OS to be age, MRD, d17p/TP53 mutation and CD49d. Mutations in ATM, BIRC3, NOTCH1 and SF3B1 were not predictive of shortened OS and many other factors routinely assessed for CLL prognostication have little value. Patients discontinuing therapy early or who experience significant infection-related toxicity have inferior outcomes and should be considered high risk for early progression.
Keyword(s): Chronic lymphocytic leukemia, Clinical outcome, Clinical trial, Prognostic groups
Abstract: EP644
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
ADMIRE and ARCTIC were phase II randomised controlled trials recruiting between July 2009-September 2012 comparing fludarabine, cyclophosphamide and rituximab (FCR) with or without mitoxantrone (FCMR) or the addition of mitoxantrone with low dose of rituximab (FCM-miniR).
Aims
To assess how pre-specified prognostic factors, minimal residual disease (MRD), toxicity and number of treatment cycles affect progression free survival (PFS) and overall survival (OS) in the combined ADMIRE/ARCTIC cohort.
Methods
ARCTIC/ADMIRE provided long-term data on 415 previously untreated CLL patients. Kaplan-Meier survival estimates, and Cox proportional hazards models assessed PFS and OS according to potential prognostic factors, toxicities and treatment cycles received. Penalised multivariable Cox regression identified the prognostic factors predictive of PFS and OS. Multiple imputation by chained equations was used to account for missing data.
Results
Median duration of follow-up is 84 months. There is no significant difference in the hazard ratios for PFS or OS between FCR and FCM-R and between FCR and FCM-miniR. Overall PFS is 65 months (95% confidence interval (CI) 56,72) and OS is 108 months (CI 101,not reached).
Univariable Cox regression analysis of PFS by pre-treatment prognostic factors revealed unmutated IGHV genes (UM-IGHV), 17p/11q deletion, TP53 mutations, CLL-IPI, %CD38+ cells, decreasing CD20 mean fluorescence intensity (MFI), %CD49d+ cells, CCR6 (MFI and %+ cells) and LAIR1 MFI to be associated with shortened PFS. A baseline multivariable penalised Cox regression analysis of PFS found UM-IGHV, CCR6, 17p/11q deletion, TP53 mutations, CD49d and SF3B1 mutations remained prognostic for PFS.
Similar univariable analysis of OS found increasing age, positive direct Coombs test, UM-IGHV, deletion 17p, TP53 mutations, CLL-IPI and %CD49d+ cells, and decreasing CCR6 MFI and LAIR1 MFI to be associated with shortened OS. Multivariable penalised Cox regression by pre-treatment factors found age, %CD49d+ cells, d17p, TP53 mutations and direct Coombs test remained prognostic for OS. Mutations in ATM, BIRC3, NOTCH1 and SF3B1 are not associated with shortened OS.
Detectable marrow MRD (>10-4) 3 months after therapy completion is associated with inferior PFS and OS on univariable analysis. Incorporation of MRD into a prognostic multivariable model revealed that UM-IGHV, MRD, d17p/TP53 mutations and %CD49d+ cells are predictive of PFS. In the same model for OS age, MRD, %CD49d+ cells, d17p/TP53 mutations and direct coombs test are predictive of OS (Table 1).
Grade 3/4 infection-related toxicities are associated with inferior PFS and OS however the occurrence of other toxicities are not associated with inferior outcomes. Hypogammaglobulinemia is not associated with the probability of grade 3/4 infection or shortened PFS/OS. Participants who received 3 cycles or less of the prescribed 6 cycles for reasons other than progressive disease had inferior PFS and OS and more likely to have other poor prognostic features.

Conclusion
A multivariable analysis of prognostic factors and MRD reveals the key prognostic factors for shortened OS to be age, MRD, d17p/TP53 mutation and CD49d. Mutations in ATM, BIRC3, NOTCH1 and SF3B1 were not predictive of shortened OS and many other factors routinely assessed for CLL prognostication have little value. Patients discontinuing therapy early or who experience significant infection-related toxicity have inferior outcomes and should be considered high risk for early progression.
Keyword(s): Chronic lymphocytic leukemia, Clinical outcome, Clinical trial, Prognostic groups


