
Contributions
Abstract: EP642
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Many patients with B-cell malignancies require continuous treatment with Bruton tyrosine kinase inhibitors (BTKi). Adverse events are a common reason for ibrutinib or acalabrutinib discontinuation. Early data from BGB-3111-215 showed zanubrutinib was well tolerated in patients with B-cell malignancies who were intolerant to either ibrutinib or acalabrutinib. Here we report preliminary results of the BGB-3111-215 trial, with a median follow-up of 4.2 months.
Aims
To assess the safety of zanubrutinib and recurrence of adverse events that led to prior BTKi intolerance.
Methods
Patients meeting protocol criteria for intolerance to ibrutinib, acalabrutinib, or both (without documented progressive disease) were given zanubrutinib monotherapy (160 mg twice daily or 320 mg once daily). Recurrence of adverse events that led to intolerance of prior BTKi and additional safety measures were assessed based on the Common Terminology Criteria for Adverse Events v5.0. Investigators determined responses using disease status at study entry as baseline and established disease criteria.
Results
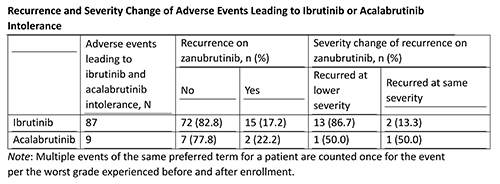
As of November 1, 2020 (data cutoff), 44 patients (n=34 chronic lymphocytic leukemia/small lymphocytic lymphoma, n=6 Waldenström macroglobulinemia, n=2 mantle cell lymphoma, n=2 marginal zone lymphoma) were enrolled, received ≥1 dose of zanubrutinib, and analyzed for safety. The median age was 70.5 y (range, 49-91); median duration of treatment was 4.2 months (range, 0.1-12.6). The median number of prior regimens was 2 (range, 1-12). Regarding prior BTKi, 39 patients received ibrutinib only, 4 received ibrutinib and acalabrutinib, and 1 received acalabrutinib only. The median number of ibrutinib- or acalabrutinib-intolerant adverse events per patient was 2 (range, 1-5). 83% of ibrutinib and 78% of acalabrutinib intolerant events did not recur on zanubrutinib; Table. At data cutoff, 43 patients remained on treatment; 1 withdrew consent due to zanubrutinib-unrelated grade 3 syncope. Overall, 34 patients (77.3%) reported any adverse event; most commonly reported adverse events were myalgia (n=9; 20.5%), contusion (n=8; 18.2%), dizziness (n=7; 15.9%), fatigue (n=7; 15.9%), and cough (n=5; 11.4%). Grade ≥3 adverse events were reported in 6 patients (13.6%), serious adverse events in 1 patient (2.3%, febrile neutropenia and salmonella infection), adverse events requiring dose interruptions in 6 patients (13.6%), and adverse events leading to dose reduction in 2 patients (4.5%). No adverse events led to zanubrutinib discontinuation. No deaths were reported. All efficacy evaluable patients (26/26 [100%]) maintained (10 [38.5%]) or achieved deepening (16 [61.5%]) of their response.
Conclusion
Zanubrutinib provided an additional treatment option after intolerance to other BTKi, demonstrating tolerability and sustained or improved efficacy. Updated results will be presented.
Keyword(s): Chronic lymphocytic leukemia, Tyrosine kinase
Abstract: EP642
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Many patients with B-cell malignancies require continuous treatment with Bruton tyrosine kinase inhibitors (BTKi). Adverse events are a common reason for ibrutinib or acalabrutinib discontinuation. Early data from BGB-3111-215 showed zanubrutinib was well tolerated in patients with B-cell malignancies who were intolerant to either ibrutinib or acalabrutinib. Here we report preliminary results of the BGB-3111-215 trial, with a median follow-up of 4.2 months.
Aims
To assess the safety of zanubrutinib and recurrence of adverse events that led to prior BTKi intolerance.
Methods
Patients meeting protocol criteria for intolerance to ibrutinib, acalabrutinib, or both (without documented progressive disease) were given zanubrutinib monotherapy (160 mg twice daily or 320 mg once daily). Recurrence of adverse events that led to intolerance of prior BTKi and additional safety measures were assessed based on the Common Terminology Criteria for Adverse Events v5.0. Investigators determined responses using disease status at study entry as baseline and established disease criteria.
Results
As of November 1, 2020 (data cutoff), 44 patients (n=34 chronic lymphocytic leukemia/small lymphocytic lymphoma, n=6 Waldenström macroglobulinemia, n=2 mantle cell lymphoma, n=2 marginal zone lymphoma) were enrolled, received ≥1 dose of zanubrutinib, and analyzed for safety. The median age was 70.5 y (range, 49-91); median duration of treatment was 4.2 months (range, 0.1-12.6). The median number of prior regimens was 2 (range, 1-12). Regarding prior BTKi, 39 patients received ibrutinib only, 4 received ibrutinib and acalabrutinib, and 1 received acalabrutinib only. The median number of ibrutinib- or acalabrutinib-intolerant adverse events per patient was 2 (range, 1-5). 83% of ibrutinib and 78% of acalabrutinib intolerant events did not recur on zanubrutinib; Table. At data cutoff, 43 patients remained on treatment; 1 withdrew consent due to zanubrutinib-unrelated grade 3 syncope. Overall, 34 patients (77.3%) reported any adverse event; most commonly reported adverse events were myalgia (n=9; 20.5%), contusion (n=8; 18.2%), dizziness (n=7; 15.9%), fatigue (n=7; 15.9%), and cough (n=5; 11.4%). Grade ≥3 adverse events were reported in 6 patients (13.6%), serious adverse events in 1 patient (2.3%, febrile neutropenia and salmonella infection), adverse events requiring dose interruptions in 6 patients (13.6%), and adverse events leading to dose reduction in 2 patients (4.5%). No adverse events led to zanubrutinib discontinuation. No deaths were reported. All efficacy evaluable patients (26/26 [100%]) maintained (10 [38.5%]) or achieved deepening (16 [61.5%]) of their response.

Conclusion
Zanubrutinib provided an additional treatment option after intolerance to other BTKi, demonstrating tolerability and sustained or improved efficacy. Updated results will be presented.
Keyword(s): Chronic lymphocytic leukemia, Tyrosine kinase


