
Contributions
Abstract: EP639
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Zanubrutinib is a highly selective, potent, and irreversible Bruton’s tyrosine kinase (BTK) inhibitor approved in China for the treatment of adult patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). The previous publication of this study reported that zanubrutinib is highly active in R/R CLL/SLL, with a well-tolerated safety profile (J Hematol Oncol. 2020;13:48). We present here the long-term results of this study.
Aims
To evaluate the efficacy, safety, and tolerability of zanubrutinib in patients with R/R CLL/SLL.
Methods
In this single-arm, multicenter, phase 2 study (NCT03206918), patients with R/R CLL/SLL received oral zanubrutinib (160 mg twice a day) continuously until progressive disease (PD) or unacceptable toxicity. Efficacy endpoints including overall response rate (ORR), duration of response (DOR), and progression-free survival (PFS) were assessed by an independent review committee (IRC) per International Workshop on CLL guidelines (Blood. 2008;111:5446) or the Lugano Classification (J Clin Oncol. 2014;32:3059) for CLL and SLL, respectively.
Results
Ninety-one patients (82 with CLL; 9 with SLL) were enrolled from 11 sites in China. Median age was 61 years (range, 35-87). Most patients had ≥1 poor prognostic variable, including unmutated immunoglobulin heavy chain variable region gene (IGHV; 56.0%), del(17p) or TP53 mutation (24.2%), and del(11q) (22%).
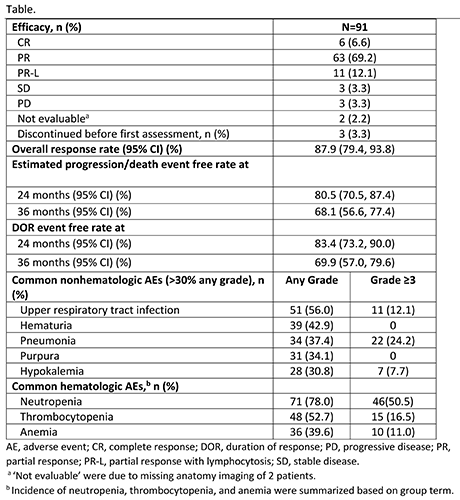
With a median 33.9-mo follow-up period (range, 0.8-41.4), 31 patients (34.1%) discontinued treatment primarily due to progressive disease (PD) in 15 patients and adverse events (AEs), regardless of relation to study drug, in 14 patients. Most patients (66%) were still on zanubrutinib treatment. The efficacy data are presented in the Table. The overall response rate was generally consistent across all subgroups analyzed, including those with unfavorable prognostic factors. Patients with del(17p) and/or TP53 mutation and del(11q) achieved high response rates of 91% (95% CI, 70.8%>98.9%) and 100% (95% CI, 83.2%>100%), respectively.
The most commonly reported treatment-emergent adverse events (TEAEs) are listed in the Table. Most AEs regarding to lab abnormalities were with low CTCAE severity (grade 1-2) and without clinical consequences. Second primary malignancies were reported in 5 patients (2 gastric adenocarcinoma; 1 each of colon cancer, breast cancer, and rectal cancer). Only 1 patient reported atrial fibrillation (grade 2). TEAEs leading to death were reported in 6 patients (2 pneumonia; 1 each for cardiopulmonary failure, brain herniation, and multiple organ dysfunction syndrome; 1 with cardiac failure, pneumonia, and respiratory failure). TEAEs leading to dose modification were reported in 42 (46.2%) patients. Commonly reported TEAEs leading to treatment discontinuation included pneumonia (n=4) and hepatitis B (n=2).
Conclusion
Results with longer follow-up continue to show a high response rate. Deep and durable responses were achieved in all patient subgroups including patients with high-risk cytogenetics. Data support the tolerability of long-term zanubrutinib treatment in R/R CLL/SLL, with no new safety signals identified.
Keyword(s): Chronic lymphocytic leukemia, Kinase inhibitor
Abstract: EP639
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Zanubrutinib is a highly selective, potent, and irreversible Bruton’s tyrosine kinase (BTK) inhibitor approved in China for the treatment of adult patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). The previous publication of this study reported that zanubrutinib is highly active in R/R CLL/SLL, with a well-tolerated safety profile (J Hematol Oncol. 2020;13:48). We present here the long-term results of this study.
Aims
To evaluate the efficacy, safety, and tolerability of zanubrutinib in patients with R/R CLL/SLL.
Methods
In this single-arm, multicenter, phase 2 study (NCT03206918), patients with R/R CLL/SLL received oral zanubrutinib (160 mg twice a day) continuously until progressive disease (PD) or unacceptable toxicity. Efficacy endpoints including overall response rate (ORR), duration of response (DOR), and progression-free survival (PFS) were assessed by an independent review committee (IRC) per International Workshop on CLL guidelines (Blood. 2008;111:5446) or the Lugano Classification (J Clin Oncol. 2014;32:3059) for CLL and SLL, respectively.
Results
Ninety-one patients (82 with CLL; 9 with SLL) were enrolled from 11 sites in China. Median age was 61 years (range, 35-87). Most patients had ≥1 poor prognostic variable, including unmutated immunoglobulin heavy chain variable region gene (IGHV; 56.0%), del(17p) or TP53 mutation (24.2%), and del(11q) (22%).
With a median 33.9-mo follow-up period (range, 0.8-41.4), 31 patients (34.1%) discontinued treatment primarily due to progressive disease (PD) in 15 patients and adverse events (AEs), regardless of relation to study drug, in 14 patients. Most patients (66%) were still on zanubrutinib treatment. The efficacy data are presented in the Table. The overall response rate was generally consistent across all subgroups analyzed, including those with unfavorable prognostic factors. Patients with del(17p) and/or TP53 mutation and del(11q) achieved high response rates of 91% (95% CI, 70.8%>98.9%) and 100% (95% CI, 83.2%>100%), respectively.
The most commonly reported treatment-emergent adverse events (TEAEs) are listed in the Table. Most AEs regarding to lab abnormalities were with low CTCAE severity (grade 1-2) and without clinical consequences. Second primary malignancies were reported in 5 patients (2 gastric adenocarcinoma; 1 each of colon cancer, breast cancer, and rectal cancer). Only 1 patient reported atrial fibrillation (grade 2). TEAEs leading to death were reported in 6 patients (2 pneumonia; 1 each for cardiopulmonary failure, brain herniation, and multiple organ dysfunction syndrome; 1 with cardiac failure, pneumonia, and respiratory failure). TEAEs leading to dose modification were reported in 42 (46.2%) patients. Commonly reported TEAEs leading to treatment discontinuation included pneumonia (n=4) and hepatitis B (n=2).

Conclusion
Results with longer follow-up continue to show a high response rate. Deep and durable responses were achieved in all patient subgroups including patients with high-risk cytogenetics. Data support the tolerability of long-term zanubrutinib treatment in R/R CLL/SLL, with no new safety signals identified.
Keyword(s): Chronic lymphocytic leukemia, Kinase inhibitor


