
Contributions
Abstract: EP636
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Ibrutinib, a once-daily Bruton’s tyrosine kinase inhibitor, is approved in the European Union and other regions for the treatment of adult patients with chronic lymphocytic leukemia (CLL); it is the only targeted therapy associated with both a significant progression-free survival (PFS) and overall survival (OS) benefit in multiple randomized phase 3 studies compared with established chemotherapy/chemoimmunotherapy regimens in patients with previously untreated CLL/small lymphocytic lymphoma (SLL).
Aims
To report extended long-term follow-up data for first-line ibrutinib vs chlorambucil in older patients with CLL/SLL from the RESONATE-2 study.
Methods
The phase 3 RESONATE-2 study enrolled patients with previously untreated CLL/SLL who were aged ≥65 years and without del(17p) (N=269). Patients were randomly assigned in a 1:1 fashion to receive once-daily single-agent ibrutinib (420 mg) until disease progression or unacceptable toxicity (n=136) or chlorambucil (0.5–0.8 mg/kg) for up to 12 cycles (n=133). Long-term outcomes included PFS, OS, overall response rate (ORR), and safety. Responses were assessed by the investigator per 2008 iwCLL criteria.
Results
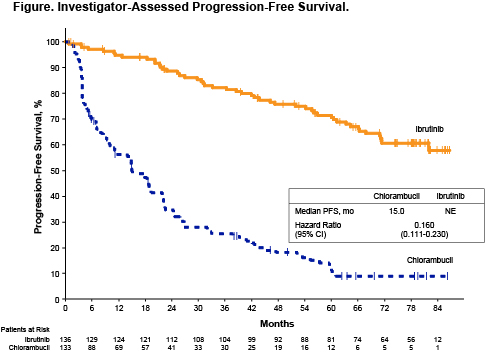
Overall, significant PFS benefit was sustained for patients treated with ibrutinib vs chlorambucil (hazard ratio [HR] 0.160 [95% confidence interval (CI) 0.111–0.230]) after up to 7 years of follow-up (median 74.9 months; range 0.1–86.8 months) (Figure). In addition to the overall population, PFS benefit with ibrutinib was observed in the subgroup of patients with high-risk genomic features, including unmutated IGHV (HR 0.109 [95% CI 0.063–0.189]) and del(11q) (HR 0.033 [95% CI 0.010–0.107]). At 6.5 years, 61% of patients treated with ibrutinib vs 9% of patients treated with chlorambucil remained progression-free. Ibrutinib treatment resulted in an OS rate of 78% at 6.5 years. The ORR for ibrutinib-treated patients was 92%, and the complete response (CR/CRi) rate rose to 34% at the current follow-up. For adverse events (AEs) of interest, ongoing rates remained low for grade ≥3 hypertension (year 5–6: 5%, n=4; year 6–7: 4%, n=3) and grade ≥3 atrial fibrillation (year 5–6: 1%, n=1; year 6–7: 1%, n=1); there were no grade ≥3 major hemorrhage events in years 5–7. Grade ≥3 AEs leading to dose reductions were observed in 1% (n=1) of patients in year 5–6 and 1% (n=1) of patients in year 6–7. For patients who had dose reductions due to any grade AEs (n=31 across years 0–8), 22/31 (71%) had resolution or improvement of the AE. Progressive disease was the primary reason for discontinuations in year 5–6 (5%, n=4) and year 6–7 (6%, n=4). AEs of any grade leading to discontinuations were observed in 3% (n=2) of patients in year 5–6 and none in year 6–7. With up to 7 years of follow-up, 47% of patients remain on single-agent ibrutinib.
Conclusion
With extended long-term follow-up from RESONATE-2, first-line treatment with ibrutinib demonstrated sustained PFS and OS benefit for patients with CLL/SLL, including patients with high-risk genomic features. Deepening of responses continued over time. Low rates of grade ≥3 AEs of interest were observed at up to 7 years of follow-up. Discontinuations and dose reductions due to AEs were rare, and most AEs leading to dose reduction resolved or improved. No new safety signals were observed, and ibrutinib continues to be well tolerated.
Keyword(s): Chlorambucil, Chronic lymphocytic leukemia, Ibrutinib, Targeted therapy
Abstract: EP636
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Ibrutinib, a once-daily Bruton’s tyrosine kinase inhibitor, is approved in the European Union and other regions for the treatment of adult patients with chronic lymphocytic leukemia (CLL); it is the only targeted therapy associated with both a significant progression-free survival (PFS) and overall survival (OS) benefit in multiple randomized phase 3 studies compared with established chemotherapy/chemoimmunotherapy regimens in patients with previously untreated CLL/small lymphocytic lymphoma (SLL).
Aims
To report extended long-term follow-up data for first-line ibrutinib vs chlorambucil in older patients with CLL/SLL from the RESONATE-2 study.
Methods
The phase 3 RESONATE-2 study enrolled patients with previously untreated CLL/SLL who were aged ≥65 years and without del(17p) (N=269). Patients were randomly assigned in a 1:1 fashion to receive once-daily single-agent ibrutinib (420 mg) until disease progression or unacceptable toxicity (n=136) or chlorambucil (0.5–0.8 mg/kg) for up to 12 cycles (n=133). Long-term outcomes included PFS, OS, overall response rate (ORR), and safety. Responses were assessed by the investigator per 2008 iwCLL criteria.
Results
Overall, significant PFS benefit was sustained for patients treated with ibrutinib vs chlorambucil (hazard ratio [HR] 0.160 [95% confidence interval (CI) 0.111–0.230]) after up to 7 years of follow-up (median 74.9 months; range 0.1–86.8 months) (Figure). In addition to the overall population, PFS benefit with ibrutinib was observed in the subgroup of patients with high-risk genomic features, including unmutated IGHV (HR 0.109 [95% CI 0.063–0.189]) and del(11q) (HR 0.033 [95% CI 0.010–0.107]). At 6.5 years, 61% of patients treated with ibrutinib vs 9% of patients treated with chlorambucil remained progression-free. Ibrutinib treatment resulted in an OS rate of 78% at 6.5 years. The ORR for ibrutinib-treated patients was 92%, and the complete response (CR/CRi) rate rose to 34% at the current follow-up. For adverse events (AEs) of interest, ongoing rates remained low for grade ≥3 hypertension (year 5–6: 5%, n=4; year 6–7: 4%, n=3) and grade ≥3 atrial fibrillation (year 5–6: 1%, n=1; year 6–7: 1%, n=1); there were no grade ≥3 major hemorrhage events in years 5–7. Grade ≥3 AEs leading to dose reductions were observed in 1% (n=1) of patients in year 5–6 and 1% (n=1) of patients in year 6–7. For patients who had dose reductions due to any grade AEs (n=31 across years 0–8), 22/31 (71%) had resolution or improvement of the AE. Progressive disease was the primary reason for discontinuations in year 5–6 (5%, n=4) and year 6–7 (6%, n=4). AEs of any grade leading to discontinuations were observed in 3% (n=2) of patients in year 5–6 and none in year 6–7. With up to 7 years of follow-up, 47% of patients remain on single-agent ibrutinib.

Conclusion
With extended long-term follow-up from RESONATE-2, first-line treatment with ibrutinib demonstrated sustained PFS and OS benefit for patients with CLL/SLL, including patients with high-risk genomic features. Deepening of responses continued over time. Low rates of grade ≥3 AEs of interest were observed at up to 7 years of follow-up. Discontinuations and dose reductions due to AEs were rare, and most AEs leading to dose reduction resolved or improved. No new safety signals were observed, and ibrutinib continues to be well tolerated.
Keyword(s): Chlorambucil, Chronic lymphocytic leukemia, Ibrutinib, Targeted therapy


