
Contributions
Abstract: EP635
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
NCCN Guidelines® provide recommendations on evolving standards of care to inform treatment (tx) decisions.
Aims
To understand the application of guidelines in routine clinical practice, we assessed how tx regimens in the informCLL registry (Mato ASH 2020) aligned with National Comprehensive Cancer Network (NCCN®)-recommended regimens for CLL/SLL.
Methods
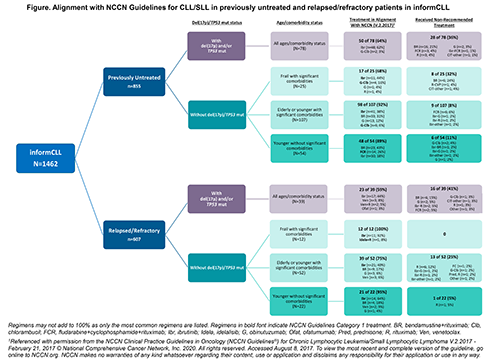
informCLL enrolled patients (pts) ≥18 years (y) receiving approved CLL tx (10/2015–6/2019) and who provided informed consent. Pts were categorized by del(17p)/TP53 mutation (mut) status, line of therapy, and age/comorbidity. Comorbidities were assessed using Charlson Comorbidity Index (CCI); significant comorbidities (SC) were defined as CCI ≥3 (Mato Blood Adv 2020). For pts without del(17p)/TP53 mut, age/comorbidity groups were: (1) frail with SC (≥65 y + CCI ≥3), (2) elderly (≥65 y + CCI <3) or younger with SC (<65 y + CCI ≥3), (3) younger without SC (<65 y + CCI <3). For this analysis, NCCN Guidelines for CLL/SLL V.2.2017 were used given 74% enrollment in ≥2017.
Results
Of 1462 pts enrolled (previously untreated [1L], 58%; relapsed/refractory [R/R], 42%), community-based practices enrolled 93%. Median age was 71 y (range 34–95); median CCI was 1.0 (range 0–11) and 16% had CCI ≥3. 27% of pts (n=389) had del(17p)/TP53 mut data available. Alignment with NCCN-recommended regimens was 59–64% for pts with del(17p)/TP53 mut and 68–100% for pts without del(17p)/TP53 mut. Most pts with del(17p)/TP53 mut (1L, n=78; R/R, n=39) had Category (Cat) 2A ibrutinib (ibr) as recommended (1L, 62%; R/R, 44%), but a quarter had non-recommended chemoimmunotherapy (CIT) with bendamustine+rituximab (BR) or fludarabine+cyclophosphamide+R (FCR) (1L, 24%; R/R, 21%). In pts without del(17p)/TP53 mut who were frail with SC (1L, n=25; R/R, n=12), 60% of 1L pts had Cat 1 ibr or obinutuzumab+chlorambucil, but 24% received BR, which was not recommended; all R/R pts had Cat 1 ibr or idelalisib+R. In pts without del(17p)/TP53 mut who were elderly/younger with SC (1L, n=107; R/R, n=52), 69% of 1L and 58% R/R pts had Cat 1 ibr or Cat 2A BR. Of pts without del(17p)/TP53 mut who were younger without SC (1L, n=54; R/R, n=22), 87% of 1L and 82% R/R pts had Cat 1 or 2A with CIT (BR or FCR), or ibr. Despite guideline recommendations for prognostic testing, a majority of pts (73%, n=1073) had no data available for del(17p)/TP53 mut status. For these pts the most common tx regimens were ibr (44%), CIT (32%), and anti-CD20 monotherapy (14%).
Conclusion
A third of pts with del(17p)/TP53 mut, a high-risk population with poor outcomes after CIT, did not receive NCCN-recommended regimens. Most pts without del(17p)/TP53 mut received recommended tx across age/comorbidity groups. A majority of pts in the registry lacked del(17p)/TP53 mut data and therefore may have received suboptimal tx. These results underscore a need for awareness of NCCN Guidelines recommendations to better inform tx decisions, especially in the community setting. References: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma V.2.2017 - February 21, 2017 © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed August 8, 2017. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Keyword(s): Chronic lymphocytic leukemia, Treatment
Abstract: EP635
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
NCCN Guidelines® provide recommendations on evolving standards of care to inform treatment (tx) decisions.
Aims
To understand the application of guidelines in routine clinical practice, we assessed how tx regimens in the informCLL registry (Mato ASH 2020) aligned with National Comprehensive Cancer Network (NCCN®)-recommended regimens for CLL/SLL.
Methods
informCLL enrolled patients (pts) ≥18 years (y) receiving approved CLL tx (10/2015–6/2019) and who provided informed consent. Pts were categorized by del(17p)/TP53 mutation (mut) status, line of therapy, and age/comorbidity. Comorbidities were assessed using Charlson Comorbidity Index (CCI); significant comorbidities (SC) were defined as CCI ≥3 (Mato Blood Adv 2020). For pts without del(17p)/TP53 mut, age/comorbidity groups were: (1) frail with SC (≥65 y + CCI ≥3), (2) elderly (≥65 y + CCI <3) or younger with SC (<65 y + CCI ≥3), (3) younger without SC (<65 y + CCI <3). For this analysis, NCCN Guidelines for CLL/SLL V.2.2017 were used given 74% enrollment in ≥2017.
Results
Of 1462 pts enrolled (previously untreated [1L], 58%; relapsed/refractory [R/R], 42%), community-based practices enrolled 93%. Median age was 71 y (range 34–95); median CCI was 1.0 (range 0–11) and 16% had CCI ≥3. 27% of pts (n=389) had del(17p)/TP53 mut data available. Alignment with NCCN-recommended regimens was 59–64% for pts with del(17p)/TP53 mut and 68–100% for pts without del(17p)/TP53 mut. Most pts with del(17p)/TP53 mut (1L, n=78; R/R, n=39) had Category (Cat) 2A ibrutinib (ibr) as recommended (1L, 62%; R/R, 44%), but a quarter had non-recommended chemoimmunotherapy (CIT) with bendamustine+rituximab (BR) or fludarabine+cyclophosphamide+R (FCR) (1L, 24%; R/R, 21%). In pts without del(17p)/TP53 mut who were frail with SC (1L, n=25; R/R, n=12), 60% of 1L pts had Cat 1 ibr or obinutuzumab+chlorambucil, but 24% received BR, which was not recommended; all R/R pts had Cat 1 ibr or idelalisib+R. In pts without del(17p)/TP53 mut who were elderly/younger with SC (1L, n=107; R/R, n=52), 69% of 1L and 58% R/R pts had Cat 1 ibr or Cat 2A BR. Of pts without del(17p)/TP53 mut who were younger without SC (1L, n=54; R/R, n=22), 87% of 1L and 82% R/R pts had Cat 1 or 2A with CIT (BR or FCR), or ibr. Despite guideline recommendations for prognostic testing, a majority of pts (73%, n=1073) had no data available for del(17p)/TP53 mut status. For these pts the most common tx regimens were ibr (44%), CIT (32%), and anti-CD20 monotherapy (14%).

Conclusion
A third of pts with del(17p)/TP53 mut, a high-risk population with poor outcomes after CIT, did not receive NCCN-recommended regimens. Most pts without del(17p)/TP53 mut received recommended tx across age/comorbidity groups. A majority of pts in the registry lacked del(17p)/TP53 mut data and therefore may have received suboptimal tx. These results underscore a need for awareness of NCCN Guidelines recommendations to better inform tx decisions, especially in the community setting. References: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma V.2.2017 - February 21, 2017 © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed August 8, 2017. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Keyword(s): Chronic lymphocytic leukemia, Treatment


