
Contributions
Abstract: EP634
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Undetectable MRD (uMRD) has become an achievable endpoint for patients (pts) with CLL, in particular using the BCL2 inhibitor VEN allowing treatment discontinuation in a MRD-driven approach. uMRD can be reached in a proportion of pts with VEN mono, and in larger fraction in combination with the BTK inhibitor IBR. It remains to be established who can achieve best responses with single agent or combination strategies.
Aims
This phase 2 multicenter MRD-driven Italian study aims at discontinuing treatment upon reaching uMRD in pts with relapsed/refractory CLL treated with VEN mono or through the addition of IBR in pts who did not achieve uMRD with the single agent.
Methods
VEN mono (up to 400 mg/day as per label) was administered for 12 months. MRD in peripheral blood (PB) and bone marrow (BM) was evaluated using the ERIC 6-color flow panel. Pts with uMRD in both PB and BM at C12D1 discontinued VEN at C12D28. Pts with detectable MRD in PB and/or BM added IBR 420 mg/day from C13D1 and continued both drugs up to C24D28, uMRD, progression or toxicity (whichever first). After C24D28, pts with detectable MRD and still in response continued IBR alone. The primary endpoint was uMRD4 (<1 CLL cell in 104 leukocytes) in both PB and BM.
Results
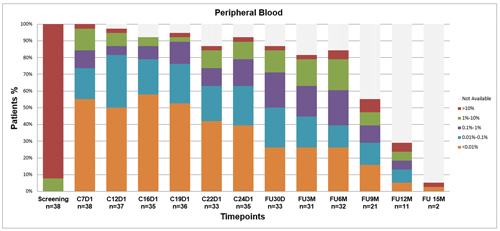
38 pts (recruited from Nov 2017 to Jul 2018) started VEN. Median number of prior therapies was 1 (range 1-4), 61% were previously treated with FC+/-R; 8/33 (24%) carried del(17p); 10/30 (33%) TP53 mutations, and 24/30 (80%) unmutated IGHV. One pt discontinued treatment due to myelodysplasia (unrelated to VEN) and 1 pt progressed on VEN mono before C12D1, 1 evaluation was missed due to COVID-19. As of 31 Jan 2021, overall response rate with VEN mono was 36/38 (95%), 19 CR and 17 PR. As per protocol, 17 pts (45%) with uMRD4 in PB and BM at C12D1 discontinued VEN at C12D28. 19 (55%) responsive cases with detectable MRD at C12D1 added IBR to VEN from C13D1. By combining IBR and VEN for a median of 7 months (range 3-10) 5/10 pts in PR improved their response to CR, 16/19 (84%) achieved uMRD4 in both PB and BM (Fig. 1), thus stopping both therapies. The remaining 3/19 continued IBR. After a median follow up of 30 months, median PFS has not been yet reached; 3 pts progressed without treatment need, 1 pt restarted VEN mono as per protocol, 2 pts developed Richter transformation. 11/33 pts (33%) who discontinued treatment in uMRD, after a median observation of 30 months remain uMRD (6 treated with VEN only). No cases of clinical and/or laboratory tumor lysis syndrome were reported in 39 pts (1 excluded from the efficacy analysis because of atypical phenotype). Adverse events were mild, with no discontinuations or dose reductions; with prolonged follow-up no new relevant toxicities occurred.
Conclusion
Our updated results demonstrated that a sequential MRD-guided approach is feasible and leads to an overall uMRD in 33/38 pts (87%) with either VEN mono or in combination with IBR. Interestingly, 84% of pts who did not achieve uMRD after VEN alone obtained uMRD after the addition of IBR and the remaining 3 patients who did not obtain uMRD even after the combination, could be selected for continuous IBR. Median PFS is not yet reached after 30 months of follow-up. This MRD-driven sequential approach allows to reach identical depth of response in each patient enrolled in the study using a personalized intensification avoiding unnecessary drug exposure, ultimately identifying the few pts that may benefit from continuous IBR. Time to clinical progression, response to VEN retreatment, and characteristics of pts with persistent MRD remain to be established.
Keyword(s): Chronic lymphocytic leukemia, Minimal residual disease (MRD), Targeted therapy
Abstract: EP634
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Undetectable MRD (uMRD) has become an achievable endpoint for patients (pts) with CLL, in particular using the BCL2 inhibitor VEN allowing treatment discontinuation in a MRD-driven approach. uMRD can be reached in a proportion of pts with VEN mono, and in larger fraction in combination with the BTK inhibitor IBR. It remains to be established who can achieve best responses with single agent or combination strategies.
Aims
This phase 2 multicenter MRD-driven Italian study aims at discontinuing treatment upon reaching uMRD in pts with relapsed/refractory CLL treated with VEN mono or through the addition of IBR in pts who did not achieve uMRD with the single agent.
Methods
VEN mono (up to 400 mg/day as per label) was administered for 12 months. MRD in peripheral blood (PB) and bone marrow (BM) was evaluated using the ERIC 6-color flow panel. Pts with uMRD in both PB and BM at C12D1 discontinued VEN at C12D28. Pts with detectable MRD in PB and/or BM added IBR 420 mg/day from C13D1 and continued both drugs up to C24D28, uMRD, progression or toxicity (whichever first). After C24D28, pts with detectable MRD and still in response continued IBR alone. The primary endpoint was uMRD4 (<1 CLL cell in 104 leukocytes) in both PB and BM.
Results
38 pts (recruited from Nov 2017 to Jul 2018) started VEN. Median number of prior therapies was 1 (range 1-4), 61% were previously treated with FC+/-R; 8/33 (24%) carried del(17p); 10/30 (33%) TP53 mutations, and 24/30 (80%) unmutated IGHV. One pt discontinued treatment due to myelodysplasia (unrelated to VEN) and 1 pt progressed on VEN mono before C12D1, 1 evaluation was missed due to COVID-19. As of 31 Jan 2021, overall response rate with VEN mono was 36/38 (95%), 19 CR and 17 PR. As per protocol, 17 pts (45%) with uMRD4 in PB and BM at C12D1 discontinued VEN at C12D28. 19 (55%) responsive cases with detectable MRD at C12D1 added IBR to VEN from C13D1. By combining IBR and VEN for a median of 7 months (range 3-10) 5/10 pts in PR improved their response to CR, 16/19 (84%) achieved uMRD4 in both PB and BM (Fig. 1), thus stopping both therapies. The remaining 3/19 continued IBR. After a median follow up of 30 months, median PFS has not been yet reached; 3 pts progressed without treatment need, 1 pt restarted VEN mono as per protocol, 2 pts developed Richter transformation. 11/33 pts (33%) who discontinued treatment in uMRD, after a median observation of 30 months remain uMRD (6 treated with VEN only). No cases of clinical and/or laboratory tumor lysis syndrome were reported in 39 pts (1 excluded from the efficacy analysis because of atypical phenotype). Adverse events were mild, with no discontinuations or dose reductions; with prolonged follow-up no new relevant toxicities occurred.

Conclusion
Our updated results demonstrated that a sequential MRD-guided approach is feasible and leads to an overall uMRD in 33/38 pts (87%) with either VEN mono or in combination with IBR. Interestingly, 84% of pts who did not achieve uMRD after VEN alone obtained uMRD after the addition of IBR and the remaining 3 patients who did not obtain uMRD even after the combination, could be selected for continuous IBR. Median PFS is not yet reached after 30 months of follow-up. This MRD-driven sequential approach allows to reach identical depth of response in each patient enrolled in the study using a personalized intensification avoiding unnecessary drug exposure, ultimately identifying the few pts that may benefit from continuous IBR. Time to clinical progression, response to VEN retreatment, and characteristics of pts with persistent MRD remain to be established.
Keyword(s): Chronic lymphocytic leukemia, Minimal residual disease (MRD), Targeted therapy


