
Contributions
Abstract: EP632
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
The CLL14 study has established fixed-duration treatment of the Bcl-2 inhibitor venetoclax and the CD20 antibody obinutuzumab (Ven-Obi) for patients (pts) with previously untreated chronic lymphocytic leukemia (CLL). High rates of undetectable minimal residual disease (uMRD) have been observed with one year of treatment.
Aims
The aim of this report is to provide a population-based exploratory analysis of MRD growth dynamics after stopping Ven-Obi and chlorambucil-Obi (Clb) therapy and to compare growth trajectories between targeted treatment versus chemoimmunotherapy.
Methods
Pts were randomized 1:1 to receive 12 cycles of Ven with 6 cycles of Obi or 12 cycles of Clb with 6 cycles of Obi. MRD was analyzed by next-generation sequencing (Adaptive clonoSEQ Assay). Samples from peripheral blood (PB) are collected every 3-6 months. For the longitudinal analyses of MRD growth dynamics, a population-based logistic growth model with nonlinear mixed effects (NLME) approach was developed to estimate population and individual patient parameters. Cases with at least two measurable timepoints were included; data < LLOQ were incorporated by likelihood-based method. Prognostic markers were screened as covariates for impact on key model parameters based on statistical (Akaike information criterion) and graphical assessments. Statistical inference on MRD doubling time and time to reach 10-2 MRD level was derived for the stratified subgroups.
Results
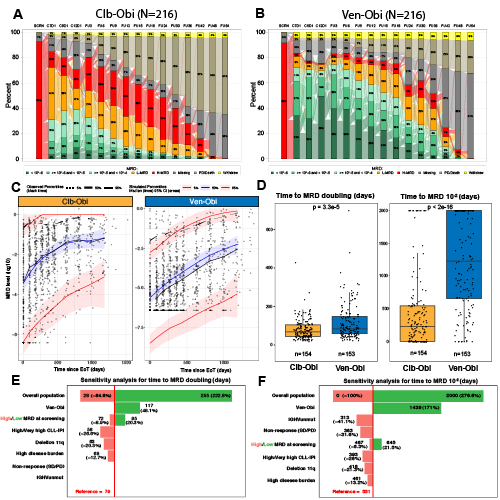
Of 432 enrolled pts, 216 were assigned to receive Clb-Obi and 216 to Ven-Obi. At follow-up month 30, 7 (3.2%) pts in the Clb-Obi arm and 58 (26.9%) pts in the Ven-Obi arm had uMRD levels <10-4 (Fig A,B). Based on the inclusion criteria for the population analysis, 154 pts from Clb-Obi arm and 153 pts from Ven-Obi arm were included. The model was well calibrated, and high concordance between observed and predicted values was confirmed (Fig C).
The median MRD level at EoT was significantly lower after Ven-Obi than after Clb-Obi (10-6.00 vs 10-3.26, p<2e-16). Within the Ven-Obi arm, end of treatment MRD values did not differ between pts with low-risk and high-risk features, such as IGHV status (10-5.79 for mutated IGHV vs 10-6.12 for unmutated IGHV) or TP53 deletion/mutation (10-5.38 for deletion/mutation vs 10-6.03 for non-deleted/mutated). The median MRD doubling time was significantly longer after Ven-Obi than Clb-Obi therapy (median days 84 versus 67 days, p = 3.3e-5)(Fig D). The median time from EoT to MRD level increase to 10-2 was also significantly longer after Ven-Obi therapy compared to Clb-Obi therapy (median 1225 days versus 227 days, p<2e-16)(Fig D).
Based on a covariate screening of 28 biological and clinical characteristics, the final model showed a significant impact on MRD growth dynamics by Ven-Obi treatment, high MRD levels at the start of treatment, high CLL-IPI, deletion11q, higher disease burden, response to treatment, and IGHV status (Fig E,F). Pts with TP53 deletion/mutation also had higher growth, although statistical significance was not reached due to low number of pts. After adjusting all the covariates in the model, the effect of Ven-Obi treatment on MRD growth remained significant (Fig E,F).
Conclusion
This analysis establishes a robust, population-based model of MRD growth dynamics that allows description of growth trajectories and treatment effects after treatment cessation. In addition to more effective MRD eradication with Ven-Obi, our results demonstrate that MRD growth is modulated more efficiently by BCL2-targeting treatment in contrast to genotoxic chemoimmunotherapy.
Keyword(s): BCL2, Chronic lymphocytic leukemia, Clonality, Minimal residual disease (MRD)
Abstract: EP632
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
The CLL14 study has established fixed-duration treatment of the Bcl-2 inhibitor venetoclax and the CD20 antibody obinutuzumab (Ven-Obi) for patients (pts) with previously untreated chronic lymphocytic leukemia (CLL). High rates of undetectable minimal residual disease (uMRD) have been observed with one year of treatment.
Aims
The aim of this report is to provide a population-based exploratory analysis of MRD growth dynamics after stopping Ven-Obi and chlorambucil-Obi (Clb) therapy and to compare growth trajectories between targeted treatment versus chemoimmunotherapy.
Methods
Pts were randomized 1:1 to receive 12 cycles of Ven with 6 cycles of Obi or 12 cycles of Clb with 6 cycles of Obi. MRD was analyzed by next-generation sequencing (Adaptive clonoSEQ Assay). Samples from peripheral blood (PB) are collected every 3-6 months. For the longitudinal analyses of MRD growth dynamics, a population-based logistic growth model with nonlinear mixed effects (NLME) approach was developed to estimate population and individual patient parameters. Cases with at least two measurable timepoints were included; data < LLOQ were incorporated by likelihood-based method. Prognostic markers were screened as covariates for impact on key model parameters based on statistical (Akaike information criterion) and graphical assessments. Statistical inference on MRD doubling time and time to reach 10-2 MRD level was derived for the stratified subgroups.
Results
Of 432 enrolled pts, 216 were assigned to receive Clb-Obi and 216 to Ven-Obi. At follow-up month 30, 7 (3.2%) pts in the Clb-Obi arm and 58 (26.9%) pts in the Ven-Obi arm had uMRD levels <10-4 (Fig A,B). Based on the inclusion criteria for the population analysis, 154 pts from Clb-Obi arm and 153 pts from Ven-Obi arm were included. The model was well calibrated, and high concordance between observed and predicted values was confirmed (Fig C).
The median MRD level at EoT was significantly lower after Ven-Obi than after Clb-Obi (10-6.00 vs 10-3.26, p<2e-16). Within the Ven-Obi arm, end of treatment MRD values did not differ between pts with low-risk and high-risk features, such as IGHV status (10-5.79 for mutated IGHV vs 10-6.12 for unmutated IGHV) or TP53 deletion/mutation (10-5.38 for deletion/mutation vs 10-6.03 for non-deleted/mutated). The median MRD doubling time was significantly longer after Ven-Obi than Clb-Obi therapy (median days 84 versus 67 days, p = 3.3e-5)(Fig D). The median time from EoT to MRD level increase to 10-2 was also significantly longer after Ven-Obi therapy compared to Clb-Obi therapy (median 1225 days versus 227 days, p<2e-16)(Fig D).
Based on a covariate screening of 28 biological and clinical characteristics, the final model showed a significant impact on MRD growth dynamics by Ven-Obi treatment, high MRD levels at the start of treatment, high CLL-IPI, deletion11q, higher disease burden, response to treatment, and IGHV status (Fig E,F). Pts with TP53 deletion/mutation also had higher growth, although statistical significance was not reached due to low number of pts. After adjusting all the covariates in the model, the effect of Ven-Obi treatment on MRD growth remained significant (Fig E,F).

Conclusion
This analysis establishes a robust, population-based model of MRD growth dynamics that allows description of growth trajectories and treatment effects after treatment cessation. In addition to more effective MRD eradication with Ven-Obi, our results demonstrate that MRD growth is modulated more efficiently by BCL2-targeting treatment in contrast to genotoxic chemoimmunotherapy.
Keyword(s): BCL2, Chronic lymphocytic leukemia, Clonality, Minimal residual disease (MRD)


