
Contributions
Abstract: EP600
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Biology & Translational Research
Background
Tumor progression locus 2 (TPL2; also known as MAP3K8) is a serine-threonine kinase that regulates innate/adaptive immunity and inflammation, through ERK activation and cytokines gene expression. Upon inflammatory signals, TPL2 modulates the expression of cytokines and chemokines that promote immune cell recruitment, differentiation, and activation, and in turn, the establishment of an inflammatory microenvironment. Increased TPL2 kinase activity is involved in inflammatory diseases and operates as a potent tumor promoter in most cancers, in association with known oncogenes. However, suppressed TPL2 expression has also been reported in some tumor types, such as lung cancer, and its ablation is associated with increased tumorigenesis in several experimental cancer models, being required for optimal p53 response to genotoxic stress. In line with the latter cases, we have observed, using gene expression datasets, that TPL2 expression is also lower in leukemic cells from patients with chronic lymphocytic leukemia (CLL) as compared to normal B cells, suggesting that TPL2 reduction might be also involved in the pathogenesis of this disease.
Aims
We aimed at investigating the in vivoeffects of TPL2 ablation in the disease development of CLL, taking advantage of the Em-TCL1 mice, a validated animal model for CLL, and at focusing our attention on the molecular pathways affected by the absence of TPL2.
Methods
We crossed TPL2-/-mice with Eμ-TCL1 mice to generate Eμ-TCL1-TPL2-/-animals. We analyzed disease expansion by assessing the percentage of CD19+CD5+malignant cells in the peripheral blood (PB), spleen, and bone marrow (BM) using flow cytometry. Malignant cells were collected from the spleen, sorted by flow cytometry, and analyzed for gene expression using RNAseq. We validated the expression levels of selected genes by qRT-PCR.
Results
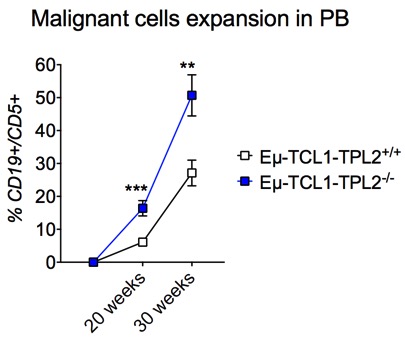
Eμ-TCL1-TPL2+/+mice develop CLL-like disease with aging. Analysis of CD19+CD5+cells at 20 and 30 weeks of age showed increased malignant cell expansion in all relevant tissues. In particular, in the PB of Eμ-TCL1-TPL2-/-mice we observed 16% malignant cells at 20 weeks and 51% at 30 weeks at variance with control Eμ-TCL1-TPL2+/+mice where malignant cells were 6% and 27%, respectively (p<0.01) (Figure 1). In the absence of TPL2, disease expansion was also accelerated in the BM (p<0.05) and spleen (p<0.01) analyzed at the same time points.
Gene expression profiling of B cells purified from Eμ-TCL1-TPL2-/-and Eμ-TCL1-TPL2+/+mice showed upregulation of genes involved in cytokine/chemokine production and leukocyte activation in the former. Validation by qRT-PCR confirmed higher expression of genes implicated in CLL pathogenesis and tissue homing in the Eμ-TCL1-TPL2-/-animals, including CCL4 (p<0.05), CCL3 (p<0.05), CCR5 (p<0.01), Zap70 (p<0.05) and BAFF (p<0.01). We also observed, in Eμ-TCL1-TPL2-/-mice, downregulation of genes involved in cell death regulation, including pro-apoptotic factors such as NOXA (p<0.01), PUMA (p<0.01), GADD45 (p<0.01), and ATF-3 (p<0.01). The expression of all these genes was not altered in either Eμ-TCL1 or TPL2-/-parental mice as compared to wild type mice.
Conclusion
Ablation of TPL2 expression accelerate CLL development in Eμ-TCL1-TPL2-/-mice. The absence of TPL2 leads to the inhibition of pro-apoptotic genes in malignant cells and the upregulation of genes with major pathogenetic roles in CLL, including tissue invasion and dissemination. Our data suggest that loss of TLP2 might synergistically contribute to disease development under pro-tumorigenic conditions.
Keyword(s): Apoptosis, Chronic lymphocytic leukemia, Signal transduction, Transgenic mice
Abstract: EP600
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Biology & Translational Research
Background
Tumor progression locus 2 (TPL2; also known as MAP3K8) is a serine-threonine kinase that regulates innate/adaptive immunity and inflammation, through ERK activation and cytokines gene expression. Upon inflammatory signals, TPL2 modulates the expression of cytokines and chemokines that promote immune cell recruitment, differentiation, and activation, and in turn, the establishment of an inflammatory microenvironment. Increased TPL2 kinase activity is involved in inflammatory diseases and operates as a potent tumor promoter in most cancers, in association with known oncogenes. However, suppressed TPL2 expression has also been reported in some tumor types, such as lung cancer, and its ablation is associated with increased tumorigenesis in several experimental cancer models, being required for optimal p53 response to genotoxic stress. In line with the latter cases, we have observed, using gene expression datasets, that TPL2 expression is also lower in leukemic cells from patients with chronic lymphocytic leukemia (CLL) as compared to normal B cells, suggesting that TPL2 reduction might be also involved in the pathogenesis of this disease.
Aims
We aimed at investigating the in vivoeffects of TPL2 ablation in the disease development of CLL, taking advantage of the Em-TCL1 mice, a validated animal model for CLL, and at focusing our attention on the molecular pathways affected by the absence of TPL2.
Methods
We crossed TPL2-/-mice with Eμ-TCL1 mice to generate Eμ-TCL1-TPL2-/-animals. We analyzed disease expansion by assessing the percentage of CD19+CD5+malignant cells in the peripheral blood (PB), spleen, and bone marrow (BM) using flow cytometry. Malignant cells were collected from the spleen, sorted by flow cytometry, and analyzed for gene expression using RNAseq. We validated the expression levels of selected genes by qRT-PCR.
Results
Eμ-TCL1-TPL2+/+mice develop CLL-like disease with aging. Analysis of CD19+CD5+cells at 20 and 30 weeks of age showed increased malignant cell expansion in all relevant tissues. In particular, in the PB of Eμ-TCL1-TPL2-/-mice we observed 16% malignant cells at 20 weeks and 51% at 30 weeks at variance with control Eμ-TCL1-TPL2+/+mice where malignant cells were 6% and 27%, respectively (p<0.01) (Figure 1). In the absence of TPL2, disease expansion was also accelerated in the BM (p<0.05) and spleen (p<0.01) analyzed at the same time points.
Gene expression profiling of B cells purified from Eμ-TCL1-TPL2-/-and Eμ-TCL1-TPL2+/+mice showed upregulation of genes involved in cytokine/chemokine production and leukocyte activation in the former. Validation by qRT-PCR confirmed higher expression of genes implicated in CLL pathogenesis and tissue homing in the Eμ-TCL1-TPL2-/-animals, including CCL4 (p<0.05), CCL3 (p<0.05), CCR5 (p<0.01), Zap70 (p<0.05) and BAFF (p<0.01). We also observed, in Eμ-TCL1-TPL2-/-mice, downregulation of genes involved in cell death regulation, including pro-apoptotic factors such as NOXA (p<0.01), PUMA (p<0.01), GADD45 (p<0.01), and ATF-3 (p<0.01). The expression of all these genes was not altered in either Eμ-TCL1 or TPL2-/-parental mice as compared to wild type mice.

Conclusion
Ablation of TPL2 expression accelerate CLL development in Eμ-TCL1-TPL2-/-mice. The absence of TPL2 leads to the inhibition of pro-apoptotic genes in malignant cells and the upregulation of genes with major pathogenetic roles in CLL, including tissue invasion and dissemination. Our data suggest that loss of TLP2 might synergistically contribute to disease development under pro-tumorigenic conditions.
Keyword(s): Apoptosis, Chronic lymphocytic leukemia, Signal transduction, Transgenic mice


