
Contributions
Abstract: EP599
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Biology & Translational Research
Background
At the 5-yr update of the Phase 3 MURANO trial (NCT02005471), median (95% CI) progression-free survival (PFS) was 54 (48–57) mo with venetoclax-rituximab (VenR) or 17 (15–22) mo with bendamustine-rituximab (BR) treatment, while 36% of VenR-treated patients (pts) and 63% of BR-treated pts had received new anti-CLL therapy. A substudy from 2018 onward enrolled 34 R/R CLL pts who progressed after initial treatment to receive VenR as retreatment (n=25) or crossover from BR (n=9). We report on the VenR-treated pts who had progressive disease (median [range] PFS 46 [36–58] mo), were eligible for anti-CLL therapy, and were retreated with VenR.
Aims
Evaluate molecular profile changes from the MURANO main study baseline (BL-1) to the retreatment baseline (BL-2), describe the genetic features at BL-2 and assess their association with MRD response to VenR.
Methods
Pts on retreatment received VenR (Ven 400mg/d for 2 yrs + monthly R for the first 6 mo). Genomic complexity (GC; defined by copy number alterations [CNA]: 0–2 non-complex, 3–4 low, ≥5 high) was assessed by array comparative genomic hybridization (aCGH). Chromosomal abnormalities were analyzed by aCGH and fluorescence in situ hybridization (high clone ≥20%, low clone 7–<20%). Peripheral blood MRD was analyzed by allele-specific oligonucleotide PCR and/or flow cytometry, with a <10-4 threshold for undetectable (u)MRD.
Results
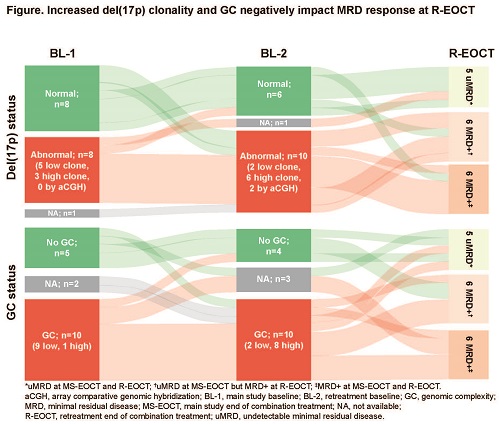
At data cutoff (13 Oct 2020), 25 pts were retreated with VenR; median (range) follow-up 12.1 (2.5–21.2) mo, median (range) treatment-free interval 28.3 (12.4–38.1) mo. Unfavorable genetic characteristics were enriched from BL-1 to BL-2. At BL-2, 14/24 pts had del(17p), including 11 high clones, with 3 newly acquired and 4 who shifted from low to high clone. An increase in CNA from BL-1 to BL-2 was observed; median (range) was 3 (1–6) at BL-1 and 4 (1–15) at BL-2 (P<0.05). Overall, 11/20 pts had GC (8 with high GC) at BL-2, with either new chromosomal abnormalities or increased clone size in poor prognostic lesions such as 2p and 8q gain, and 7q, 8p, 11q and 18p loss.
Investigator-assessed objective response rate to VenR retreatment was 70% (14/20 evaluable pts; 50% [14/25] among intent-to-treat pts; 5 complete response, 9 partial response, 5 non-evaluable). Of 17 pts with available MRD data at the retreatment end of combination treatment (R-EOCT), 5 achieved uMRD and 12 were MRD+ (Figure). Sixteen pts had evaluable del(17p) results. Of 6 pts without del(17p) at BL-2, 4 attained uMRD at R-EOCT while all 10 with del(17p) at BL-2 were MRD+ at R-EOCT. Of these, 4/10 had new del(17p) from BL-1 or had evolved from del(17p) low clone to high clone. GC status was available for 14 pts. Of 4 pts without GC at BL-2, 2 attained uMRD at R-EOCT. Of 10 pts with GC at BL-2, 8 were MRD+ and 2 had uMRD at R-EOCT. Of the pts with GC at BL-2, 5/10 had new or increased GC from BL-1. Ten pts attained uMRD at main study EOCT; 4/10 re-attained uMRD at R-EOCT and 6/10 did not. Among the 6 who were unable to re-attain uMRD, 83% (5/6) had del(17p) at BL-2 (3/5 were newly evolved from BL-1) compared with 40% (2/5) at BL-1.
Conclusion
Pts who relapsed after initial VenR therapy were highly enriched for unfavorable characteristics at BL-2. We observed a genetic profile shift from BL-1 to BL-2, with greater GC as well as higher prevalence and increased clonality in del(17p). Increased prevalence of unfavorable genetic risk factors negatively impacted MRD response of retreatment pts at the R-EOCT. Deep initial response alongside favorable BL-2 characteristics predict attainment of uMRD with VenR retreatment.
Keyword(s): Chronic lymphocytic leukemia, Gene deletion, Minimal residual disease (MRD), Retreatment
Abstract: EP599
Type: E-Poster Presentation
Session title: Chronic lymphocytic leukemia and related disorders - Biology & Translational Research
Background
At the 5-yr update of the Phase 3 MURANO trial (NCT02005471), median (95% CI) progression-free survival (PFS) was 54 (48–57) mo with venetoclax-rituximab (VenR) or 17 (15–22) mo with bendamustine-rituximab (BR) treatment, while 36% of VenR-treated patients (pts) and 63% of BR-treated pts had received new anti-CLL therapy. A substudy from 2018 onward enrolled 34 R/R CLL pts who progressed after initial treatment to receive VenR as retreatment (n=25) or crossover from BR (n=9). We report on the VenR-treated pts who had progressive disease (median [range] PFS 46 [36–58] mo), were eligible for anti-CLL therapy, and were retreated with VenR.
Aims
Evaluate molecular profile changes from the MURANO main study baseline (BL-1) to the retreatment baseline (BL-2), describe the genetic features at BL-2 and assess their association with MRD response to VenR.
Methods
Pts on retreatment received VenR (Ven 400mg/d for 2 yrs + monthly R for the first 6 mo). Genomic complexity (GC; defined by copy number alterations [CNA]: 0–2 non-complex, 3–4 low, ≥5 high) was assessed by array comparative genomic hybridization (aCGH). Chromosomal abnormalities were analyzed by aCGH and fluorescence in situ hybridization (high clone ≥20%, low clone 7–<20%). Peripheral blood MRD was analyzed by allele-specific oligonucleotide PCR and/or flow cytometry, with a <10-4 threshold for undetectable (u)MRD.
Results
At data cutoff (13 Oct 2020), 25 pts were retreated with VenR; median (range) follow-up 12.1 (2.5–21.2) mo, median (range) treatment-free interval 28.3 (12.4–38.1) mo. Unfavorable genetic characteristics were enriched from BL-1 to BL-2. At BL-2, 14/24 pts had del(17p), including 11 high clones, with 3 newly acquired and 4 who shifted from low to high clone. An increase in CNA from BL-1 to BL-2 was observed; median (range) was 3 (1–6) at BL-1 and 4 (1–15) at BL-2 (P<0.05). Overall, 11/20 pts had GC (8 with high GC) at BL-2, with either new chromosomal abnormalities or increased clone size in poor prognostic lesions such as 2p and 8q gain, and 7q, 8p, 11q and 18p loss.
Investigator-assessed objective response rate to VenR retreatment was 70% (14/20 evaluable pts; 50% [14/25] among intent-to-treat pts; 5 complete response, 9 partial response, 5 non-evaluable). Of 17 pts with available MRD data at the retreatment end of combination treatment (R-EOCT), 5 achieved uMRD and 12 were MRD+ (Figure). Sixteen pts had evaluable del(17p) results. Of 6 pts without del(17p) at BL-2, 4 attained uMRD at R-EOCT while all 10 with del(17p) at BL-2 were MRD+ at R-EOCT. Of these, 4/10 had new del(17p) from BL-1 or had evolved from del(17p) low clone to high clone. GC status was available for 14 pts. Of 4 pts without GC at BL-2, 2 attained uMRD at R-EOCT. Of 10 pts with GC at BL-2, 8 were MRD+ and 2 had uMRD at R-EOCT. Of the pts with GC at BL-2, 5/10 had new or increased GC from BL-1. Ten pts attained uMRD at main study EOCT; 4/10 re-attained uMRD at R-EOCT and 6/10 did not. Among the 6 who were unable to re-attain uMRD, 83% (5/6) had del(17p) at BL-2 (3/5 were newly evolved from BL-1) compared with 40% (2/5) at BL-1.

Conclusion
Pts who relapsed after initial VenR therapy were highly enriched for unfavorable characteristics at BL-2. We observed a genetic profile shift from BL-1 to BL-2, with greater GC as well as higher prevalence and increased clonality in del(17p). Increased prevalence of unfavorable genetic risk factors negatively impacted MRD response of retreatment pts at the R-EOCT. Deep initial response alongside favorable BL-2 characteristics predict attainment of uMRD with VenR retreatment.
Keyword(s): Chronic lymphocytic leukemia, Gene deletion, Minimal residual disease (MRD), Retreatment


