
Contributions
Abstract: EP596
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Due to complement-associated defects and the use of C5 inhibitors, the risk of infection may be high in patients with paroxysmal nocturnal hemoglobinuria (PNH). However, there are only limited data on comparative rates of hospitalized serious infections (SI) in patients (pts) with PNH vs pts without PNH.
Aims
To compare the incidence rate (IR) of SI, including bacterial, viral, or opportunistic infections in pts with or without PNH in a real-world setting.
Methods
Using the IBM MarketScan Commercial Database, a cohort of pts with PNH was identified with confirmed diagnosis of PNH between Jan 1, 2000 and Dec 31, 2019 and ≥3 claims for PNH within 365 consecutive days. The earliest claim that satisfied this criterion was assigned as the index. A cohort of individuals with no evidence of PNH in the study period was randomly selected from the database and matched (without replacement) to the PNH cohort by age, sex, insurance type, region, enrollment length, HIV status, diabetes status, and chemotherapy status at a 1:3 ratio. In this analysis, cases and controls were followed up for ≥6 months. Cases of SI were identified via validated algorithms (Pawar 2019 [PMID: 30679153]; Dommasch 2019 [PMID: 31075163]; Khan 2020 [PMID: 30980714]). We limited our analysis to SI to reduce surveillance bias associated with assessing outpatient infections. The analysis used all infections with discharge diagnosis codes, and a sensitivity analysis was performed by restricting to only SI with primary discharge diagnosis code. The SI discharge dates were required to be separated by ≥28 days to reduce the risk of counting readmissions for same infection (Feldman 2017 [PMID: 27589220]). A sensitivity analysis was performed using ≥14 days. Mean cumulative function (MCF) was used to characterize the cumulative number of infections. A Poisson regression model was fitted to estimate the incidence rate ratio (IRR) and 95% CI. The hazard of first infection was compared between groups via Cox proportional hazards model. Subgroup analyses were performed for infection subtypes and for pts with PNH with or without aplastic anemia (AA).
Results
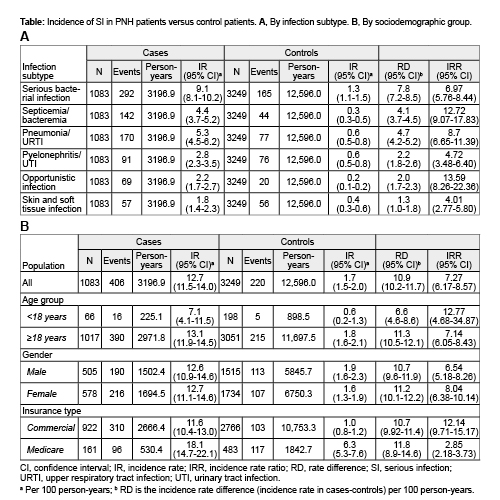
Overall, 1083 pts with PNH (200 [18.5%] with AA; 322 [29.7%] who received anti-C5 agents during follow-up) and 3249 control pts were identified. In all, 20.9% of pts with PNH and 4.7% of control pts experienced ≥1 SI during a follow-up of 3196.9 and 12,596.0 person-years, respectively. The IR of SI was 12.7 (95% CI: 11.5, 14.0) and 1.7 (95% CI: 1.5, 2.0) per 100 person-years for pts with PNH and control pts, respectively, translating into an IRR of 7.27 (95% CI: 6.17, 8.57). The overall IR for SI was not significantly different between PNH patients with or without AA. Subgroup analyses showed higher IR in pts with PNH compared with control pts across all infection subtypes (Fig. 1A) and sex and age groups (Fig. 1B). The hazard of first infection was higher in pts with PNH vs control pts (HR, 6.08; 95% CI: 4.95, 7.48). The MCFs suggested that for every 100 pts with PNH, there were 15, 26, and 57 excess SI compared with control pts within 1, 2, and 5 years, respectively. The IR did not change when the allowable gap between discharge dates was ≥14 days. The higher IR of SI in pts with PNH remained consistent when the analysis was restricted to only SI with primary discharge diagnosis status (IRR, 6.75; 95% CI: 5.16, 8.84).
Conclusion
This analysis revealed substantial burden of SI in pts with PNH compared with pts without PNH. Measures to minimize the risk of SI could improve health outcomes of pts with PNH and reduce infection-related healthcare costs.
Keyword(s): Complement, Health care, Infection, Paroxysmal nocturnal hemoglobinuria (PNH)
Abstract: EP596
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Due to complement-associated defects and the use of C5 inhibitors, the risk of infection may be high in patients with paroxysmal nocturnal hemoglobinuria (PNH). However, there are only limited data on comparative rates of hospitalized serious infections (SI) in patients (pts) with PNH vs pts without PNH.
Aims
To compare the incidence rate (IR) of SI, including bacterial, viral, or opportunistic infections in pts with or without PNH in a real-world setting.
Methods
Using the IBM MarketScan Commercial Database, a cohort of pts with PNH was identified with confirmed diagnosis of PNH between Jan 1, 2000 and Dec 31, 2019 and ≥3 claims for PNH within 365 consecutive days. The earliest claim that satisfied this criterion was assigned as the index. A cohort of individuals with no evidence of PNH in the study period was randomly selected from the database and matched (without replacement) to the PNH cohort by age, sex, insurance type, region, enrollment length, HIV status, diabetes status, and chemotherapy status at a 1:3 ratio. In this analysis, cases and controls were followed up for ≥6 months. Cases of SI were identified via validated algorithms (Pawar 2019 [PMID: 30679153]; Dommasch 2019 [PMID: 31075163]; Khan 2020 [PMID: 30980714]). We limited our analysis to SI to reduce surveillance bias associated with assessing outpatient infections. The analysis used all infections with discharge diagnosis codes, and a sensitivity analysis was performed by restricting to only SI with primary discharge diagnosis code. The SI discharge dates were required to be separated by ≥28 days to reduce the risk of counting readmissions for same infection (Feldman 2017 [PMID: 27589220]). A sensitivity analysis was performed using ≥14 days. Mean cumulative function (MCF) was used to characterize the cumulative number of infections. A Poisson regression model was fitted to estimate the incidence rate ratio (IRR) and 95% CI. The hazard of first infection was compared between groups via Cox proportional hazards model. Subgroup analyses were performed for infection subtypes and for pts with PNH with or without aplastic anemia (AA).
Results
Overall, 1083 pts with PNH (200 [18.5%] with AA; 322 [29.7%] who received anti-C5 agents during follow-up) and 3249 control pts were identified. In all, 20.9% of pts with PNH and 4.7% of control pts experienced ≥1 SI during a follow-up of 3196.9 and 12,596.0 person-years, respectively. The IR of SI was 12.7 (95% CI: 11.5, 14.0) and 1.7 (95% CI: 1.5, 2.0) per 100 person-years for pts with PNH and control pts, respectively, translating into an IRR of 7.27 (95% CI: 6.17, 8.57). The overall IR for SI was not significantly different between PNH patients with or without AA. Subgroup analyses showed higher IR in pts with PNH compared with control pts across all infection subtypes (Fig. 1A) and sex and age groups (Fig. 1B). The hazard of first infection was higher in pts with PNH vs control pts (HR, 6.08; 95% CI: 4.95, 7.48). The MCFs suggested that for every 100 pts with PNH, there were 15, 26, and 57 excess SI compared with control pts within 1, 2, and 5 years, respectively. The IR did not change when the allowable gap between discharge dates was ≥14 days. The higher IR of SI in pts with PNH remained consistent when the analysis was restricted to only SI with primary discharge diagnosis status (IRR, 6.75; 95% CI: 5.16, 8.84).

Conclusion
This analysis revealed substantial burden of SI in pts with PNH compared with pts without PNH. Measures to minimize the risk of SI could improve health outcomes of pts with PNH and reduce infection-related healthcare costs.
Keyword(s): Complement, Health care, Infection, Paroxysmal nocturnal hemoglobinuria (PNH)


