
Contributions
Abstract: EP594
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Patients (pts) with paroxysmal nocturnal hemoglobinuria (PNH) often present with chronic intravascular hemolysis and clinical sequelae due to uncontrolled terminal complement activation, leading to an increased risk of thrombosis and death. In two phase 3 randomized trials of pts with PNH, ravulizumab (administered every 8 weeks; q8w) was shown to be non-inferior to eculizumab (every 2 weeks; q2w) for all efficacy endpoints with a similar safety profile and no unexpected safety signals. Each study had a 26-week randomized primary evaluation period followed by an extension period during which all pts received ravulizumab. Safety results from the 27 weeks–2-years extension period for both studies were analyzed.
Aims
Describe the 2-year safety profile of ravulizumab in pts with PNH from two phase 3 trials.
Methods
Clinical trials 301 (NCT02946463) and 302 (NCT03056040) were phase 3, randomized, open-label, noninferiority, multicenter studies of adult pts with confirmed diagnosis of PNH. Pts in study 301 were naïve to complement C5 inhibitor therapy, with lactate dehydrogenase (LDH) levels ≥ 1.5 × ULN and ≥ 1 sign or symptom of PNH at screening, and generally referred to as pts with high disease activity. Pts in study 302 had been stable on eculizumab treatment for ≥ 6 months prior to study entry, with LDH levels ≤ 1.5 × ULN at screening. Pts received weight-based dosing of ravulizumab q8w or the approved eculizumab dose (900 mg, q2w) for 26 weeks; eligible pts could continue on, or switch to, ravulizumab in the extension period. The incidence of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), breakthrough hemolysis (BTH) and anti-drug antibody (ADA) status were assessed in the extension period.
Results
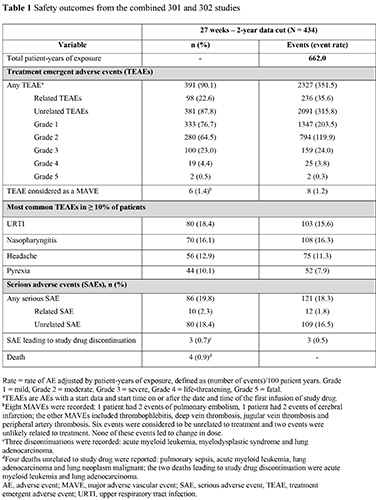
Patient demographics were similar across both studies. In total for combined studies, 434 pts received ravulizumab treatment in the extension period. Of these, 214 were complement C5 inhibitor-experienced having received eculizumab prior to switching to ravulizumab in the extension period. Total exposure for ravulizumab (27 weeks–2-years) was 662 patient-years for the studies. Ninety-eight pts (22.6%) reported treatment-related TEAEs, 10 pts (2.3%) reported treatment-related SAEs, and 3 pts (0.7%) had an SAE that led to study drug discontinuation (unrelated to treatment). Six pts (1.4%) had a major adverse vascular event (MAVE). The most commonly reported TEAEs (in ≥ 10% of pts) were upper respiratory tract infection, nasopharyngitis, headache and pyrexia, n (%): 80 (18.4); 70 (16.1); 56 (12.9); 44 (10.1), respectively (Table 1). The percentage of pts with BTH events in the 18-month follow-up extension periods were similar with 15 (6.2%) and 11 (5.6%) in pts naïve to complement C5 inhibitor therapy (study 301) and those with prior eculizumab use (study 302), respectively. Immunogenicity at 2 years was low across both studies, with treatment-emergent non-neutralizing ADA-positivity in 5 pts. Four deaths unrelated to treatment were reported during the extension period. Meningococcal infection was not observed up to the 2-year extension period; there was one fatal case of meningococcal sepsis in a patient at 2.2 years after receiving first dose of ravulizumab. More than 90% of pts in both studies continued ravulizumab treatment beyond the 2-year data cut of the extension periods.
Conclusion
Ravulizumab was well tolerated in pts with PNH who were complement C5 inhibitor therapy naïve or experienced, with no new safety signals reported up to 2 years of treatment.
Keyword(s): Adverse reaction, Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Safety
Abstract: EP594
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Patients (pts) with paroxysmal nocturnal hemoglobinuria (PNH) often present with chronic intravascular hemolysis and clinical sequelae due to uncontrolled terminal complement activation, leading to an increased risk of thrombosis and death. In two phase 3 randomized trials of pts with PNH, ravulizumab (administered every 8 weeks; q8w) was shown to be non-inferior to eculizumab (every 2 weeks; q2w) for all efficacy endpoints with a similar safety profile and no unexpected safety signals. Each study had a 26-week randomized primary evaluation period followed by an extension period during which all pts received ravulizumab. Safety results from the 27 weeks–2-years extension period for both studies were analyzed.
Aims
Describe the 2-year safety profile of ravulizumab in pts with PNH from two phase 3 trials.
Methods
Clinical trials 301 (NCT02946463) and 302 (NCT03056040) were phase 3, randomized, open-label, noninferiority, multicenter studies of adult pts with confirmed diagnosis of PNH. Pts in study 301 were naïve to complement C5 inhibitor therapy, with lactate dehydrogenase (LDH) levels ≥ 1.5 × ULN and ≥ 1 sign or symptom of PNH at screening, and generally referred to as pts with high disease activity. Pts in study 302 had been stable on eculizumab treatment for ≥ 6 months prior to study entry, with LDH levels ≤ 1.5 × ULN at screening. Pts received weight-based dosing of ravulizumab q8w or the approved eculizumab dose (900 mg, q2w) for 26 weeks; eligible pts could continue on, or switch to, ravulizumab in the extension period. The incidence of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), breakthrough hemolysis (BTH) and anti-drug antibody (ADA) status were assessed in the extension period.
Results
Patient demographics were similar across both studies. In total for combined studies, 434 pts received ravulizumab treatment in the extension period. Of these, 214 were complement C5 inhibitor-experienced having received eculizumab prior to switching to ravulizumab in the extension period. Total exposure for ravulizumab (27 weeks–2-years) was 662 patient-years for the studies. Ninety-eight pts (22.6%) reported treatment-related TEAEs, 10 pts (2.3%) reported treatment-related SAEs, and 3 pts (0.7%) had an SAE that led to study drug discontinuation (unrelated to treatment). Six pts (1.4%) had a major adverse vascular event (MAVE). The most commonly reported TEAEs (in ≥ 10% of pts) were upper respiratory tract infection, nasopharyngitis, headache and pyrexia, n (%): 80 (18.4); 70 (16.1); 56 (12.9); 44 (10.1), respectively (Table 1). The percentage of pts with BTH events in the 18-month follow-up extension periods were similar with 15 (6.2%) and 11 (5.6%) in pts naïve to complement C5 inhibitor therapy (study 301) and those with prior eculizumab use (study 302), respectively. Immunogenicity at 2 years was low across both studies, with treatment-emergent non-neutralizing ADA-positivity in 5 pts. Four deaths unrelated to treatment were reported during the extension period. Meningococcal infection was not observed up to the 2-year extension period; there was one fatal case of meningococcal sepsis in a patient at 2.2 years after receiving first dose of ravulizumab. More than 90% of pts in both studies continued ravulizumab treatment beyond the 2-year data cut of the extension periods.

Conclusion
Ravulizumab was well tolerated in pts with PNH who were complement C5 inhibitor therapy naïve or experienced, with no new safety signals reported up to 2 years of treatment.
Keyword(s): Adverse reaction, Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Safety


