
Contributions
Abstract: EP590
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Paroxysmal nocturnal hemoglobinuria (PNH) typically manifests during adulthood, with pediatric patients accounting for ~14% of reported cases. Ravulizumab is a terminal complement component 5 (C5) inhibitor shown to have a similar efficacy and safety profile to eculizumab in adults with PNH, and a study in pediatric patients is ongoing.
Aims
To assess the pharmacokinetics (PK), pharmacodynamics (PD), efficacy and safety of ravulizumab in a pediatric population with PNH.
Methods
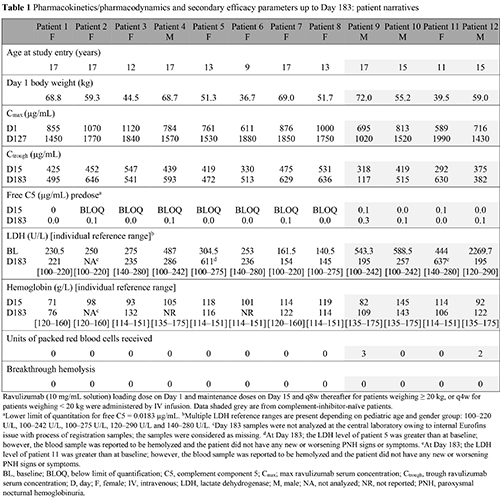
This ongoing phase 3, multicenter, single-arm, open-label study (NCT03406507) enrolled complement-inhibitor-naïve and eculizumab-experienced pediatric patients (aged < 18 years) with a diagnosis of PNH, at 9 sites in 6 countries. Naïve patients received a weight-based loading dose (LD) of ravulizumab on Day 1 followed by weight-based maintenance dose (MD) on Day 15, and then once every 8 weeks [LD (mg)/MD (mg)]: ≥ 30 to < 40 kg bodyweight [1200 mg/2700 mg]; ≥ 40 to < 60 kg bodyweight [2400/3000]; ≥ 60 to < 100 kg bodyweight [2700/3300]. For patients switching from eculizumab therapy, ravulizumab Day 1 was 2 weeks after the last eculizumab dose. All analyses were performed to the end of the primary evaluation period (Day 183). Primary endpoints included ravulizumab maximum and trough serum concentration (Cmax, Ctrough; at the end of dosing interval at steady state), accumulation ratio and change in free C5 concentration. Secondary endpoints included percentage change in lactate dehydrogenase (LDH) from baseline, transfusion avoidance (TA), breakthrough hemolysis (BTH) and hemoglobin stabilization (Hgb-S). Safety endpoints included incidence of treatment-emergent adverse events (TEAE) and serious adverse events (SAE).
Results
In total, 13 patients were enrolled, 12 (4 complement-inhibitor-naïve) were included in the interim analysis (1 patient did not complete Day 183 evaluation by database lock: 27 May 2020; Table 1). Ten (83.3%) patients were aged ≥ 12 years, with a median (range) age at first ravulizumab infusion of 15.0 (9–17) years. Steady-state therapeutic serum concentrations of ravulizumab were achieved immediately following the first dose and maintained throughout the primary evaluation period with no evidence of accumulation (mean [SD] Cmax and Ctrough accumulation ratios of 1.1 [0.1] and 1.1 [0.2], respectively). Complete terminal complement inhibition was attained by the end of first ravulizumab infusion (defined as mean serum free C5 concentration < 0.5 μg/mL) and was sustained through Day 183 in all patients (Table 1). Mean (SD) percentage change in LDH was –42.1% (59.0) in complement-inhibitor-naïve patients and remained stable in eculizumab-experienced patients at +4.65% (44.7). Overall, 10 (83.3%) patients achieved TA, 8 (66.7%) achieved Hgb-S and no patients experienced BTH. TEAE were reported in 10 patients; the most frequent were abdominal pain and nasopharyngitis. No meningococcal infections, deaths or discontinuations due to TEAE were reported. Three patients had ≥ 1 SAE during the study and no SAE were assessed to be treatment-related.
Conclusion
These interim results are from the largest study of pediatric patients with PNH to date. In this rare population, ravulizumab was effective, well-tolerated, and provided immediate, complete, and sustained terminal complement inhibition irrespective of prior treatment with eculizumab. These PK, PD, efficacy and safety findings suggest that pediatric patients can be initiated on ravulizumab or switched from eculizumab to ravulizumab.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Pediatric, Pharmacokinetic
Abstract: EP590
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Paroxysmal nocturnal hemoglobinuria (PNH) typically manifests during adulthood, with pediatric patients accounting for ~14% of reported cases. Ravulizumab is a terminal complement component 5 (C5) inhibitor shown to have a similar efficacy and safety profile to eculizumab in adults with PNH, and a study in pediatric patients is ongoing.
Aims
To assess the pharmacokinetics (PK), pharmacodynamics (PD), efficacy and safety of ravulizumab in a pediatric population with PNH.
Methods
This ongoing phase 3, multicenter, single-arm, open-label study (NCT03406507) enrolled complement-inhibitor-naïve and eculizumab-experienced pediatric patients (aged < 18 years) with a diagnosis of PNH, at 9 sites in 6 countries. Naïve patients received a weight-based loading dose (LD) of ravulizumab on Day 1 followed by weight-based maintenance dose (MD) on Day 15, and then once every 8 weeks [LD (mg)/MD (mg)]: ≥ 30 to < 40 kg bodyweight [1200 mg/2700 mg]; ≥ 40 to < 60 kg bodyweight [2400/3000]; ≥ 60 to < 100 kg bodyweight [2700/3300]. For patients switching from eculizumab therapy, ravulizumab Day 1 was 2 weeks after the last eculizumab dose. All analyses were performed to the end of the primary evaluation period (Day 183). Primary endpoints included ravulizumab maximum and trough serum concentration (Cmax, Ctrough; at the end of dosing interval at steady state), accumulation ratio and change in free C5 concentration. Secondary endpoints included percentage change in lactate dehydrogenase (LDH) from baseline, transfusion avoidance (TA), breakthrough hemolysis (BTH) and hemoglobin stabilization (Hgb-S). Safety endpoints included incidence of treatment-emergent adverse events (TEAE) and serious adverse events (SAE).
Results
In total, 13 patients were enrolled, 12 (4 complement-inhibitor-naïve) were included in the interim analysis (1 patient did not complete Day 183 evaluation by database lock: 27 May 2020; Table 1). Ten (83.3%) patients were aged ≥ 12 years, with a median (range) age at first ravulizumab infusion of 15.0 (9–17) years. Steady-state therapeutic serum concentrations of ravulizumab were achieved immediately following the first dose and maintained throughout the primary evaluation period with no evidence of accumulation (mean [SD] Cmax and Ctrough accumulation ratios of 1.1 [0.1] and 1.1 [0.2], respectively). Complete terminal complement inhibition was attained by the end of first ravulizumab infusion (defined as mean serum free C5 concentration < 0.5 μg/mL) and was sustained through Day 183 in all patients (Table 1). Mean (SD) percentage change in LDH was –42.1% (59.0) in complement-inhibitor-naïve patients and remained stable in eculizumab-experienced patients at +4.65% (44.7). Overall, 10 (83.3%) patients achieved TA, 8 (66.7%) achieved Hgb-S and no patients experienced BTH. TEAE were reported in 10 patients; the most frequent were abdominal pain and nasopharyngitis. No meningococcal infections, deaths or discontinuations due to TEAE were reported. Three patients had ≥ 1 SAE during the study and no SAE were assessed to be treatment-related.

Conclusion
These interim results are from the largest study of pediatric patients with PNH to date. In this rare population, ravulizumab was effective, well-tolerated, and provided immediate, complete, and sustained terminal complement inhibition irrespective of prior treatment with eculizumab. These PK, PD, efficacy and safety findings suggest that pediatric patients can be initiated on ravulizumab or switched from eculizumab to ravulizumab.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Pediatric, Pharmacokinetic


