
Contributions
Abstract: EP586
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The intravenous (IV) complement C5 inhibitors eculizumab and ravulizumab are the current standard of care for patients (pts) with paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) where available. Ravulizumab self-administered via a subcutaneous (SC) on-body delivery system will offer an alternative to ravulizumab IV and allow administration outside of clinic which can improve patient independence.
Aims
Evaluate the pharmacokinetic (PK) non-inferiority of ravulizumab SC versus ravulizumab IV in adult pts with PNH.
Methods
This ongoing phase 3, multicenter, randomized, open-label study (NCT03748823) enrolled clinically stable (lactate dehydrogenase [LDH] level ≤ 1.5 upper limit of normal) adult pts (aged ≥ 18 years) with PNH treated with eculizumab for ≥ 3 months prior to entry. In the 10-week primary evaluation period, pts received a weight-based loading dose of ravulizumab IV on Day 1. On Day 15, pts on ravulizumab IV received a single weight-based maintenance dose of ravulizumab, and those randomized to ravulizumab SC received a 490 mg maintenance dose and then every week (qw) thereafter. During the extension phase (up to 3.5 years), all pts will receive ravulizumab SC qw. Primary endpoint was Day 71 serum ravulizumab predose concentration (Day 71 Ctrough). Non-inferiority was determined between the two groups using a one-sided test at an α level of 0.05, and a PK non-inferiority boundary of 0.8. Secondary endpoints included Ctrough and free serum C5 concentration over time and incidence of anti-drug antibodies (ADA). Efficacy indicators were change in LDH level, breakthrough hemolysis (BTH), hemoglobin stabilization (Hgb-S), transfusion avoidance (TA) and PNH symptoms. Safety indicators included adverse device events (ADE), serious ADE (SADE), adverse events (AE) and serious AE (SAE).
Results
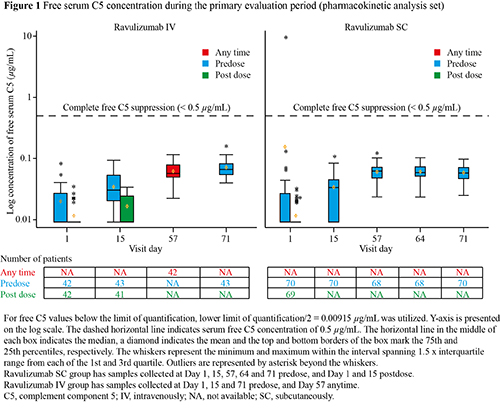
This primary analysis of PK non-inferiority of ravulizumab SC versus IV administration included 70 pts in the SC group and 43 pts in the IV group. Patient demographics were comparable between groups. Day 71 geometric mean Ctrough concentration for ravulizumab SC demonstrated PK non-inferiority with ravulizumab IV (559.9 µg/ml and 445.3 µg/mL, respectively; ratio of geometric least squares means SC/IV: 1.26 [90% CI: 1.16–1.36; p < 0.0001]). All Ctrough levels were above target trough serum concentration, and no pts tested positive for ADA at Day 71. Complete and sustained suppression of serum free C5 (mean concentration < 0.5 µg/mL) was achieved in both groups at all timepoints after the first dose (Figure 1). Mean (SD) percentage change in LDH from baseline was 1.7% (34.8%) and 5.1% (29.6%) in pts on ravulizumab SC and IV, respectively. Only 1 BTH event due to unknown cause was reported in a patient on ravulizumab IV. Hgb-S was achieved by 93.6% and 81.8% of pts, and TA was achieved by 94.0% and 86.7% of pts on ravulizumab SC and IV, respectively. PNH symptoms were observed in 45.2% and 46.7% of pts on ravulizumab SC and IV, respectively. Pts on ravulizumab SC with ≥ 1 ADE were 45.2% (23.8% device use), and no SADE were reported. Proportion of pts experiencing ≥ 1 AE were comparable between -ravulizumab SC and IV when ADE were excluded. Five (6.0%) pts on ravulizumab SC experienced ≥ 1 SAE versus 1 patient (2.2%) on ravulizumab IV; none were related to study treatment.
Conclusion
Ravulizumab SC Ctrough levels were non-inferior to those obtained with ravulizumab IV. Ravulizumab SC provided complete and sustained free C5 inhibition comparable to ravulizumab IV at all timepoints.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Pharmacokinetic, Subcutaneous
Abstract: EP586
Type: E-Poster Presentation
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The intravenous (IV) complement C5 inhibitors eculizumab and ravulizumab are the current standard of care for patients (pts) with paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) where available. Ravulizumab self-administered via a subcutaneous (SC) on-body delivery system will offer an alternative to ravulizumab IV and allow administration outside of clinic which can improve patient independence.
Aims
Evaluate the pharmacokinetic (PK) non-inferiority of ravulizumab SC versus ravulizumab IV in adult pts with PNH.
Methods
This ongoing phase 3, multicenter, randomized, open-label study (NCT03748823) enrolled clinically stable (lactate dehydrogenase [LDH] level ≤ 1.5 upper limit of normal) adult pts (aged ≥ 18 years) with PNH treated with eculizumab for ≥ 3 months prior to entry. In the 10-week primary evaluation period, pts received a weight-based loading dose of ravulizumab IV on Day 1. On Day 15, pts on ravulizumab IV received a single weight-based maintenance dose of ravulizumab, and those randomized to ravulizumab SC received a 490 mg maintenance dose and then every week (qw) thereafter. During the extension phase (up to 3.5 years), all pts will receive ravulizumab SC qw. Primary endpoint was Day 71 serum ravulizumab predose concentration (Day 71 Ctrough). Non-inferiority was determined between the two groups using a one-sided test at an α level of 0.05, and a PK non-inferiority boundary of 0.8. Secondary endpoints included Ctrough and free serum C5 concentration over time and incidence of anti-drug antibodies (ADA). Efficacy indicators were change in LDH level, breakthrough hemolysis (BTH), hemoglobin stabilization (Hgb-S), transfusion avoidance (TA) and PNH symptoms. Safety indicators included adverse device events (ADE), serious ADE (SADE), adverse events (AE) and serious AE (SAE).
Results
This primary analysis of PK non-inferiority of ravulizumab SC versus IV administration included 70 pts in the SC group and 43 pts in the IV group. Patient demographics were comparable between groups. Day 71 geometric mean Ctrough concentration for ravulizumab SC demonstrated PK non-inferiority with ravulizumab IV (559.9 µg/ml and 445.3 µg/mL, respectively; ratio of geometric least squares means SC/IV: 1.26 [90% CI: 1.16–1.36; p < 0.0001]). All Ctrough levels were above target trough serum concentration, and no pts tested positive for ADA at Day 71. Complete and sustained suppression of serum free C5 (mean concentration < 0.5 µg/mL) was achieved in both groups at all timepoints after the first dose (Figure 1). Mean (SD) percentage change in LDH from baseline was 1.7% (34.8%) and 5.1% (29.6%) in pts on ravulizumab SC and IV, respectively. Only 1 BTH event due to unknown cause was reported in a patient on ravulizumab IV. Hgb-S was achieved by 93.6% and 81.8% of pts, and TA was achieved by 94.0% and 86.7% of pts on ravulizumab SC and IV, respectively. PNH symptoms were observed in 45.2% and 46.7% of pts on ravulizumab SC and IV, respectively. Pts on ravulizumab SC with ≥ 1 ADE were 45.2% (23.8% device use), and no SADE were reported. Proportion of pts experiencing ≥ 1 AE were comparable between -ravulizumab SC and IV when ADE were excluded. Five (6.0%) pts on ravulizumab SC experienced ≥ 1 SAE versus 1 patient (2.2%) on ravulizumab IV; none were related to study treatment.

Conclusion
Ravulizumab SC Ctrough levels were non-inferior to those obtained with ravulizumab IV. Ravulizumab SC provided complete and sustained free C5 inhibition comparable to ravulizumab IV at all timepoints.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Pharmacokinetic, Subcutaneous


