
Contributions
Abstract: EP579
Type: E-Poster Presentation
Session title: Bleeding disorders (congenital and acquired)
Background
Von Willebrand disease (VWD) is a disorder predominantly attributable to a deficiency or dysfunction of the Von Willebrand factor (VWF) glycoprotein. It is typically characterized by recurrent episodes of mucocutaneous bleeding. Gastrointestinal (GI) bleeding associated with angiectasy is a rare but challenging complication which can result in frequent hospitalizations for red cell transfusion. Management options have been limited to off license use of anti-angiogenic and hormonal agents lacking supporting data. Since the discovery of elevated vascular endothelial growth factor (VEGF) expression in patients with VWD, reports of Bevacizumab, a monoclonal antibody against VEGF, in managing complications of VWD have emerged, with most of the reported experience in recurrent epistaxis. We present a case of VWD with refractory and progressive transfusion requirements secondary to severe GI bleeding that did not respond to conventional therapy.
Aims
Present a case of VWD with refractory transfusion requirements secondary to severe GI bleeding.
Methods
Clinical case report.
Results
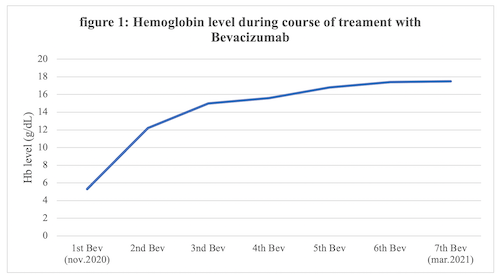
A 60-year-old man with Type IIA VWD chronically medicated with VWF/FVIII prophylaxis and recurrent epistaxis, presented recurrent GI bleeding associated with small intestine angiectasies. Treatment with VWF/FVIII prophylaxis and octreotide resulted in only temporary cessation of bleeding and the patient had enduring transfusion weekly requirements with frequent need for hospitalization. Despite endoscopic and medical treatment attempts with hormonal agents and octreotide, the patient developed persistent hemorrhage and severe anemia, leading to red blood cell transfusions dependency. Subsequently he commenced on a course of Bevacizumab (5mg/kg, every 15 days). Immediately after initiating Bevacizumab, complete cessation of bleeding episodes was observed and hemoglobin levels anticipated a growing trend, as demonstrated in figure 1. The patient has been transfusion-independent since beginning anti-VEGF therapy. Currently, after 4 months of follow-up, he maintained sustained complete response without the occurrence of adverse events.
Conclusion
Our patient shown to gain noteworthy benefit from anti-VEGF therapy (Bevacizumab) with total transfusion independence and significant improvement in quality of life. This case illustrates the challenging nature of angiodysplasia-related GI bleeding in patients with VWD and shows that Bevacizumab seems to be a safe, well tolerated and efficient treatment in this group of patients. Nevertheless, the optimal dosage and duration of bevacizumab for GI bleeding in VWD, remains to be determined, and requires balancing the clinical benefits against potential adverse events of systemic treatment such as hypertension and thromboembolic events.
Keyword(s): Anti-angiogenesis, Bleeding disorder, Von Willebrand's disease
Abstract: EP579
Type: E-Poster Presentation
Session title: Bleeding disorders (congenital and acquired)
Background
Von Willebrand disease (VWD) is a disorder predominantly attributable to a deficiency or dysfunction of the Von Willebrand factor (VWF) glycoprotein. It is typically characterized by recurrent episodes of mucocutaneous bleeding. Gastrointestinal (GI) bleeding associated with angiectasy is a rare but challenging complication which can result in frequent hospitalizations for red cell transfusion. Management options have been limited to off license use of anti-angiogenic and hormonal agents lacking supporting data. Since the discovery of elevated vascular endothelial growth factor (VEGF) expression in patients with VWD, reports of Bevacizumab, a monoclonal antibody against VEGF, in managing complications of VWD have emerged, with most of the reported experience in recurrent epistaxis. We present a case of VWD with refractory and progressive transfusion requirements secondary to severe GI bleeding that did not respond to conventional therapy.
Aims
Present a case of VWD with refractory transfusion requirements secondary to severe GI bleeding.
Methods
Clinical case report.
Results
A 60-year-old man with Type IIA VWD chronically medicated with VWF/FVIII prophylaxis and recurrent epistaxis, presented recurrent GI bleeding associated with small intestine angiectasies. Treatment with VWF/FVIII prophylaxis and octreotide resulted in only temporary cessation of bleeding and the patient had enduring transfusion weekly requirements with frequent need for hospitalization. Despite endoscopic and medical treatment attempts with hormonal agents and octreotide, the patient developed persistent hemorrhage and severe anemia, leading to red blood cell transfusions dependency. Subsequently he commenced on a course of Bevacizumab (5mg/kg, every 15 days). Immediately after initiating Bevacizumab, complete cessation of bleeding episodes was observed and hemoglobin levels anticipated a growing trend, as demonstrated in figure 1. The patient has been transfusion-independent since beginning anti-VEGF therapy. Currently, after 4 months of follow-up, he maintained sustained complete response without the occurrence of adverse events.

Conclusion
Our patient shown to gain noteworthy benefit from anti-VEGF therapy (Bevacizumab) with total transfusion independence and significant improvement in quality of life. This case illustrates the challenging nature of angiodysplasia-related GI bleeding in patients with VWD and shows that Bevacizumab seems to be a safe, well tolerated and efficient treatment in this group of patients. Nevertheless, the optimal dosage and duration of bevacizumab for GI bleeding in VWD, remains to be determined, and requires balancing the clinical benefits against potential adverse events of systemic treatment such as hypertension and thromboembolic events.
Keyword(s): Anti-angiogenesis, Bleeding disorder, Von Willebrand's disease


