
Contributions
Abstract: EP575
Type: E-Poster Presentation
Session title: Bleeding disorders (congenital and acquired)
Background
Despite major advances in the treatment of hemophilia A, limitations experienced by patients still remain. Good clinical treatment outcomes such as low annualized bleeding rates depend on careful patient adherence to the regimen of their factor VIII (FVIII) product. Another important aspect of patient self-management is portability and storage of recombinant FVIII (rFVIII) products. Patients with hemophilia A can benefit from the use of rFVIII products such as turoctocog alfa and turoctocog alfa pegol since, unlike other products, they can be stored at temperatures ≤40°C for ≤3 months.
Aims
To identify limitations regarding compliance, storage conditions and understanding of treatment with FVIII products among patients with hemophilia A.
Methods
Patients with hemophilia A in Slovenia who use different FVIII products completed a 10-minute, self-administered questionnaire comprising 22 questions. Questions focusing on patients’ hemophilia burden, everyday limitations, their perceptions and understanding of FVIII product storage were included.
Results
Data were collected between February and July 2020. Of 63 individuals surveyed (n=61 patients, n=2 caregivers), most were >18 years old (59 patients, 94%), had severe hemophilia A (43 patients, 68%), were treated prophylactically (44 out of 60 respondents, 73%) and dosed 3 times per week (19 out of 37 respondents, 51%) with different FVIII products. Mean (median) numbers of bleeds within the current 12 months were 5.7 (3.0) and 2.3 (1.0) for prophylactically and episodically treated patients, respectively, due to severe disease for patients on prophylaxis. Almost half of the respondents (26 out of 56 respondents, 46%) reported missed or forgotten doses. Of the 21 prophylactically treated patients who had their pharmacokinetics (PK) assessed, the reported trough FVIII activity ranged from 0-24%.
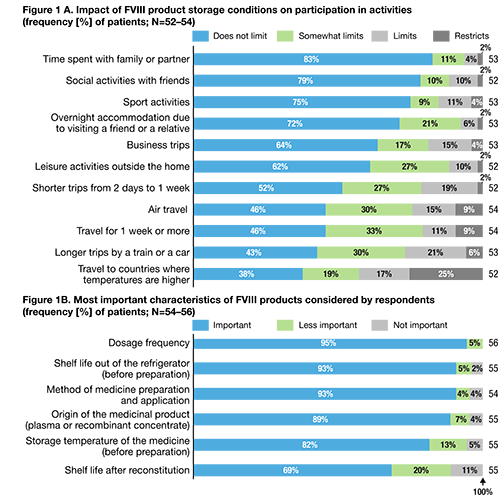
Bleed frequency within the last 12 months was higher for patients reporting missed/forgotten doses (mean [median], 5.9 [4.0]) compared to those patients with no missed/forgotten doses (4.6 [3.0]) during the same period. Most patients (42 out of 53 respondents, 79%) stored their product in a refrigerator and believed that FVIII products should always be stored at ≤25°C (49 out of 54 respondents, 91%). Perceived storage conditions limited daily activities for 76% (42 out of 55 respondents) of patients; travel (business trips and work activities) was the activity most frequently impacted (Figure 1A). Forty-six patients (73%) indicated that treatment satisfaction would be improved by a FVIII product that could be stored at >25°C (reasons included improved convenience [29 respondents, 63%], improved quality of life [9 respondents, 20%] and fewer restrictions on activities [8 respondents, 17%]). Interestingly, there was no difference between younger and older patients. Most patients considered dosing frequency, shelf life out of the refrigerator and method of product preparation/application as the most important characteristics of their FVIII product (Figure 1B).
Conclusion
Patients of all ages with hemophilia A often feel restricted in their daily activities by the perceived need to store FVIII products at <25°C. These restrictions have an impact on adherence to treatment. From the patients’ perspective, the most important FVIII product characteristics were dosage frequency followed by shelf life out of refrigerator. Improved patient awareness and understanding of FVIII treatment could improve compliance and treatment outcomes in patients with hemophilia A.
Keyword(s): Clinical outcome, Factor VIII, Hemophilia A, Practice
Abstract: EP575
Type: E-Poster Presentation
Session title: Bleeding disorders (congenital and acquired)
Background
Despite major advances in the treatment of hemophilia A, limitations experienced by patients still remain. Good clinical treatment outcomes such as low annualized bleeding rates depend on careful patient adherence to the regimen of their factor VIII (FVIII) product. Another important aspect of patient self-management is portability and storage of recombinant FVIII (rFVIII) products. Patients with hemophilia A can benefit from the use of rFVIII products such as turoctocog alfa and turoctocog alfa pegol since, unlike other products, they can be stored at temperatures ≤40°C for ≤3 months.
Aims
To identify limitations regarding compliance, storage conditions and understanding of treatment with FVIII products among patients with hemophilia A.
Methods
Patients with hemophilia A in Slovenia who use different FVIII products completed a 10-minute, self-administered questionnaire comprising 22 questions. Questions focusing on patients’ hemophilia burden, everyday limitations, their perceptions and understanding of FVIII product storage were included.
Results
Data were collected between February and July 2020. Of 63 individuals surveyed (n=61 patients, n=2 caregivers), most were >18 years old (59 patients, 94%), had severe hemophilia A (43 patients, 68%), were treated prophylactically (44 out of 60 respondents, 73%) and dosed 3 times per week (19 out of 37 respondents, 51%) with different FVIII products. Mean (median) numbers of bleeds within the current 12 months were 5.7 (3.0) and 2.3 (1.0) for prophylactically and episodically treated patients, respectively, due to severe disease for patients on prophylaxis. Almost half of the respondents (26 out of 56 respondents, 46%) reported missed or forgotten doses. Of the 21 prophylactically treated patients who had their pharmacokinetics (PK) assessed, the reported trough FVIII activity ranged from 0-24%.
Bleed frequency within the last 12 months was higher for patients reporting missed/forgotten doses (mean [median], 5.9 [4.0]) compared to those patients with no missed/forgotten doses (4.6 [3.0]) during the same period. Most patients (42 out of 53 respondents, 79%) stored their product in a refrigerator and believed that FVIII products should always be stored at ≤25°C (49 out of 54 respondents, 91%). Perceived storage conditions limited daily activities for 76% (42 out of 55 respondents) of patients; travel (business trips and work activities) was the activity most frequently impacted (Figure 1A). Forty-six patients (73%) indicated that treatment satisfaction would be improved by a FVIII product that could be stored at >25°C (reasons included improved convenience [29 respondents, 63%], improved quality of life [9 respondents, 20%] and fewer restrictions on activities [8 respondents, 17%]). Interestingly, there was no difference between younger and older patients. Most patients considered dosing frequency, shelf life out of the refrigerator and method of product preparation/application as the most important characteristics of their FVIII product (Figure 1B).

Conclusion
Patients of all ages with hemophilia A often feel restricted in their daily activities by the perceived need to store FVIII products at <25°C. These restrictions have an impact on adherence to treatment. From the patients’ perspective, the most important FVIII product characteristics were dosage frequency followed by shelf life out of refrigerator. Improved patient awareness and understanding of FVIII treatment could improve compliance and treatment outcomes in patients with hemophilia A.
Keyword(s): Clinical outcome, Factor VIII, Hemophilia A, Practice


