
Contributions
Abstract: EP567
Type: E-Poster Presentation
Session title: Bleeding disorders (congenital and acquired)
Background
Patients with non-severe hemophilia A can perioperatively be treated with desmopressin or factor VIII (FVIII) concentrate. Desmopressin produces an increase of FVIII plasma levels. However, interpatient variability in the FVIII response is high. If the response is sufficient, minor procedures can be performed with desmopressin only. In case the response is insufficient, FVIII concentrate needs to be administered. The 2020 WFH guideline states that this combination therapy can overcome downsides associated with use of only DDAVP or FVIII concentrate. However, treatment of desmopressin combined with FVIII concentrate is not common practice and difficult to implement. Furthermore, as desmopressin is less expensive than FVIII concentrate, combination therapy may lead to considerable FVIII savings and would be useful in case of limited FVIII concentrate resources.
Aims
To assess accuracy, safety and FVIII concentrate savings of peri-operative pharmacokinetic (PK)-guided dosing of desmopressin combined with FVIII concentrate in non-severe hemophilia patients.
Methods
Hemophilia A patients responsive to desmopressin undergoing a procedure were included. Desmopressin was administered intravenously (0.3 µg/kg) once a day. Based on previously determined FVIII response on desmopressin, additional FVIII concentrate dosing was calculated using PK-guidance. Modelling technique was continuously adapted and improved based on study data. Desmopressin was administered for a maximum of three consecutive days. FVIII activity was determined before and after combination therapy (trough and peak levels). Hypothetical FVIII concentrate loading dose was calculated, assuming an increase of 0.02 IU/ml per IU FVIII concentrate per kilogram bodyweight. A predicted FVIII level by the desmopressin-FVIII PK-model was considered accurate if difference between predicted and measured FVIII level was ≤ 0.2 IU/ml.
Results
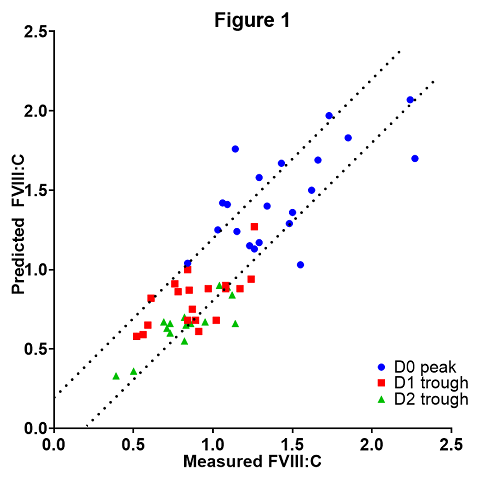
Twenty-one procedures were performed in 20 hemophilia A patients, of whom 19 mild and 1 moderate. Median age was 47 years (interquartile range (IQR) 40 - 59). Model predictions were accurate in 13/21 (61.9%) pre-operative peak levels at day 0 (D0, day of surgery), 14/19 (73.7%) at day 1 (D1) and 13/17 (76.5%) at day 2 (D2) post-operative trough levels. Figure 1 describes model accuracy, comparing measured FVIII levels with predicted FVIII levels and with dotted lines signifying +/- 0.2 IU/ml. FVIII concentrate loading dose per patient was 1250 IU (median; IQR 1000 - 1750) lower than hypothetical loading dose. Two patients had bleeding complications, with both patients having supraphysiological (>1.0 IU/ml) FVIII activity at the time of bleeding.
Conclusion
Peri-operative combination therapy of desmopressin and FVIII concentrate under PK guidance in non-severe hemophilia A patients provides a feasible and accurate treatment in the majority of patients. This novel approach resulted in considerable FVIII concentrate savings.
Keyword(s): Factor VIII, Hemophilia A, Treatment
Abstract: EP567
Type: E-Poster Presentation
Session title: Bleeding disorders (congenital and acquired)
Background
Patients with non-severe hemophilia A can perioperatively be treated with desmopressin or factor VIII (FVIII) concentrate. Desmopressin produces an increase of FVIII plasma levels. However, interpatient variability in the FVIII response is high. If the response is sufficient, minor procedures can be performed with desmopressin only. In case the response is insufficient, FVIII concentrate needs to be administered. The 2020 WFH guideline states that this combination therapy can overcome downsides associated with use of only DDAVP or FVIII concentrate. However, treatment of desmopressin combined with FVIII concentrate is not common practice and difficult to implement. Furthermore, as desmopressin is less expensive than FVIII concentrate, combination therapy may lead to considerable FVIII savings and would be useful in case of limited FVIII concentrate resources.
Aims
To assess accuracy, safety and FVIII concentrate savings of peri-operative pharmacokinetic (PK)-guided dosing of desmopressin combined with FVIII concentrate in non-severe hemophilia patients.
Methods
Hemophilia A patients responsive to desmopressin undergoing a procedure were included. Desmopressin was administered intravenously (0.3 µg/kg) once a day. Based on previously determined FVIII response on desmopressin, additional FVIII concentrate dosing was calculated using PK-guidance. Modelling technique was continuously adapted and improved based on study data. Desmopressin was administered for a maximum of three consecutive days. FVIII activity was determined before and after combination therapy (trough and peak levels). Hypothetical FVIII concentrate loading dose was calculated, assuming an increase of 0.02 IU/ml per IU FVIII concentrate per kilogram bodyweight. A predicted FVIII level by the desmopressin-FVIII PK-model was considered accurate if difference between predicted and measured FVIII level was ≤ 0.2 IU/ml.
Results
Twenty-one procedures were performed in 20 hemophilia A patients, of whom 19 mild and 1 moderate. Median age was 47 years (interquartile range (IQR) 40 - 59). Model predictions were accurate in 13/21 (61.9%) pre-operative peak levels at day 0 (D0, day of surgery), 14/19 (73.7%) at day 1 (D1) and 13/17 (76.5%) at day 2 (D2) post-operative trough levels. Figure 1 describes model accuracy, comparing measured FVIII levels with predicted FVIII levels and with dotted lines signifying +/- 0.2 IU/ml. FVIII concentrate loading dose per patient was 1250 IU (median; IQR 1000 - 1750) lower than hypothetical loading dose. Two patients had bleeding complications, with both patients having supraphysiological (>1.0 IU/ml) FVIII activity at the time of bleeding.

Conclusion
Peri-operative combination therapy of desmopressin and FVIII concentrate under PK guidance in non-severe hemophilia A patients provides a feasible and accurate treatment in the majority of patients. This novel approach resulted in considerable FVIII concentrate savings.
Keyword(s): Factor VIII, Hemophilia A, Treatment


