
Contributions
Abstract: EP554
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Methotrexate (MTX) is an antimetabolite, that mainly acts through the inhibition of the enzyme dihydrofolate reductase and the subsequent depletion of intracellular folate stores. High-dose MTX (HDMTX)is defined by the dose of more than 1 g/m2. The utilization of HDMTX is commonly used in solid malignancies such as osteosarcoma and hematological malignancies such as acute lymphoblastic leukemia and non-Hodgkin’s Lymphoma. Urine Alkalinization using intravenous sodium bicarbonate and calcium folinate to facilitate clearance of MTX are crucial two steps in MTX clearance and to avoid or minimize side effects such as nephrotoxicity and hepatotoxicity. For that reason, most institutions are requiring patients to be admitted with a minimum length of stay of 72 hours until methotrexate clearance is achieved with a serum level of < 0.1 micromol/L.
Aims
Here, we are assessing the safety of ambulatory HDMTX among adult patients. Regular laboratory monitoring, adequate oral hydration and oral medications were utilized to enhance methotrexate clearance without the necessity to use devices at home for infusion or admitting the patient
Methods
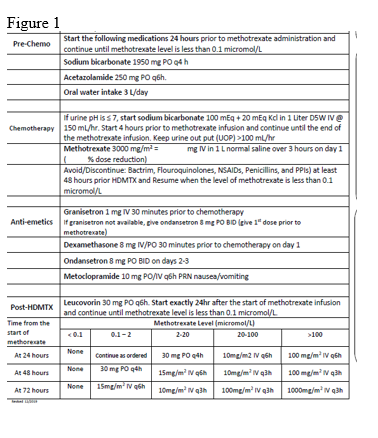
This trial is open-labeled, single arm study that aims to evaluate the safety of high dose methotrexate administration in an outpatient setting among adult patients with hematological malignancies in a tertiary care center. The study aims to cover a cohort of patients with hematological malignancies from 14 to 60-years-old treated at KFMC scheduled to receive HDMTX from (February-2020) to (February-2021). treatment protocol is showing in figure 1.
Results
Twenty-seven (n=27) cycles were done until now. Around 70.37% of the patients were diagnosed with diffuse large B cell lymphoma (DLBCL).
Thirteen patients (48.1) % of the patients achieved MTX level less than 0.1 micromol/L by 48 hours and 24 patients (88.8%) achieved the same level by 72 hours.
Only three (11.1%) patients did not clear their methotrexate by the end of 72 hours; one patient was admitted due to acute kidney injury, the second was managed as outpatient as that occurred at the mid of COVID-19 bed crises, and the third one refused admission and was following his labs at the hospital at his hometown. By 72 hours, 29.63% had reversible asymptomatic elevation of creatinine, and all of them were grade one.
By 72 hours, around 44.44% had reversible asymptomatic hepatotoxicity, and all of them were grade 1. Among the 27 patients, only one suffered from acute kidney injury that necessitated admission for supportive care, which was totally reversible. The patient was not compliant to the treatment protocol and was excluded for subsequent cycles.
Conclusion
Our preliminary results suggest that ambulatory HDMTX is safe, cost effective, more comfortable for patients.
These results encourage the use of this approach more frequently as it decreases hospitalization and thus reduces cost, increases patient satisfaction and helps to solve the issue of bed crisis.
Keyword(s): Follow-up, Malignant lymphoma, Safety, Treatment
Abstract: EP554
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Methotrexate (MTX) is an antimetabolite, that mainly acts through the inhibition of the enzyme dihydrofolate reductase and the subsequent depletion of intracellular folate stores. High-dose MTX (HDMTX)is defined by the dose of more than 1 g/m2. The utilization of HDMTX is commonly used in solid malignancies such as osteosarcoma and hematological malignancies such as acute lymphoblastic leukemia and non-Hodgkin’s Lymphoma. Urine Alkalinization using intravenous sodium bicarbonate and calcium folinate to facilitate clearance of MTX are crucial two steps in MTX clearance and to avoid or minimize side effects such as nephrotoxicity and hepatotoxicity. For that reason, most institutions are requiring patients to be admitted with a minimum length of stay of 72 hours until methotrexate clearance is achieved with a serum level of < 0.1 micromol/L.
Aims
Here, we are assessing the safety of ambulatory HDMTX among adult patients. Regular laboratory monitoring, adequate oral hydration and oral medications were utilized to enhance methotrexate clearance without the necessity to use devices at home for infusion or admitting the patient
Methods
This trial is open-labeled, single arm study that aims to evaluate the safety of high dose methotrexate administration in an outpatient setting among adult patients with hematological malignancies in a tertiary care center. The study aims to cover a cohort of patients with hematological malignancies from 14 to 60-years-old treated at KFMC scheduled to receive HDMTX from (February-2020) to (February-2021). treatment protocol is showing in figure 1.
Results
Twenty-seven (n=27) cycles were done until now. Around 70.37% of the patients were diagnosed with diffuse large B cell lymphoma (DLBCL).
Thirteen patients (48.1) % of the patients achieved MTX level less than 0.1 micromol/L by 48 hours and 24 patients (88.8%) achieved the same level by 72 hours.
Only three (11.1%) patients did not clear their methotrexate by the end of 72 hours; one patient was admitted due to acute kidney injury, the second was managed as outpatient as that occurred at the mid of COVID-19 bed crises, and the third one refused admission and was following his labs at the hospital at his hometown. By 72 hours, 29.63% had reversible asymptomatic elevation of creatinine, and all of them were grade one.
By 72 hours, around 44.44% had reversible asymptomatic hepatotoxicity, and all of them were grade 1. Among the 27 patients, only one suffered from acute kidney injury that necessitated admission for supportive care, which was totally reversible. The patient was not compliant to the treatment protocol and was excluded for subsequent cycles.

Conclusion
Our preliminary results suggest that ambulatory HDMTX is safe, cost effective, more comfortable for patients.
These results encourage the use of this approach more frequently as it decreases hospitalization and thus reduces cost, increases patient satisfaction and helps to solve the issue of bed crisis.
Keyword(s): Follow-up, Malignant lymphoma, Safety, Treatment


