
Contributions
Abstract: EP553
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
L-MIND (NCT02399085) is an ongoing, open-label, single-arm, Phase II study of tafasitamab + LEN in patients (pts) with R/R DLBCL who are ineligible for ASCT. Progression-free survival (PFS) strongly correlates with overall survival (OS); however, while PFS estimates study drug treatment effect, OS also includes effects of subsequent cancer treatments or palliative care. Given that not all OS and PFS events may occur even during a long follow-up, traditional survival analyses may underestimate survival benefit for tafasitamab + LEN by assuming the same mortality rate for the whole study population.
Aims
To estimate the proportion of long-term survivors (LTS) and survival benefit associated with tafasitamab + LEN treatment using mixture cure models.
Methods
In the L-MIND study (data cut-off: Oct 30, 2020), 80/81 enrolled pts with R/R DLBCL received tafasitamab + LEN, with a median and maximum follow-up duration of 42.7 months (95% confidence interval (CI): 38.0–47.2) and 54.6 months, respectively. Kaplan–Meier (KM) plots of PFS and OS were observed to plateau after 30 months, with the majority of events (PFS: 45/46 [97.8%]; OS: 36/41 [87.8%]) reported up to that time-point. LTS exhibit a high survival benefit from tafasitamab + LEN treatment and were defined as pts without a PFS event and who did not decease after the observed plateau. Thus, the pt population can be considered to consist of two sub-populations: LTS and non-LTS. A ‘mixture cure model’ was fitted on L-MIND observed PFS and OS data to estimate the proportion of LTS and associated mean survival for the two sub-populations. To incorporate background mortality, age- and sex-specific US mortality rates were factored in for the entire study population (enrolled in Europe and US). The weighted average of the mean survival for the two sub-populations provided an estimate of the mean survival for the whole population. For comparative purposes, standard parametric models without considering an LTS proportion were also fitted on PFS and OS data. Sensitivity analyses were performed considering different survival distributions (exponential distribution, Weibull distribution and log-logistic distribution).
Results
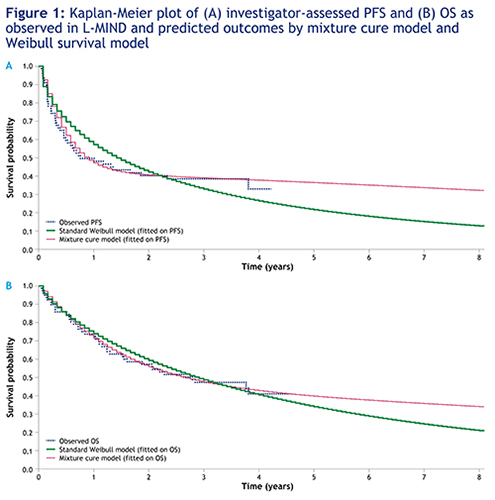
Using a mixture cure model fitted to PFS and OS data, the estimated proportion of LTS for the tafasitamab + LEN combination was 41.7% (95% CI: 30.0–54.5) and 44.6% (95% CI: 28.1–62.4), respectively. The predicted mean survival when the mixture cure model was fitted on PFS data was 16.7 years for LTS pts vs 0.5 years for non-LTS pts, yielding 7.4 years for the overall population. Further, survival rates for pts with tafasitamab + LEN treatment were predicted as 40.7% and 36.5% at 2 and 5 years, respectively. Similar results were obtained when the mixture cure model was fitted on OS data. By comparison, using a standard parametric model (Weibull distribution), the predicted mean survival in the overall population was 3.6 years fitting PFS data (Figure 1A) and 5.2 years fitting OS data (Figure 1B).
Conclusion
PFS and OS KM curves for the L-MIND study reaching a long plateau and not following a standard Weibull distribution suggest the presence of an LTS subgroup. Standard parametric models may fail to capture the survival plateau of LTS, leading to a poor estimate of OS benefit in such pts. The mixture cure model suggests that the treatment of R/R DLBCL pts with tafasitamab + LEN is associated with a high LTS proportion of 41.7%.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody
Abstract: EP553
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
L-MIND (NCT02399085) is an ongoing, open-label, single-arm, Phase II study of tafasitamab + LEN in patients (pts) with R/R DLBCL who are ineligible for ASCT. Progression-free survival (PFS) strongly correlates with overall survival (OS); however, while PFS estimates study drug treatment effect, OS also includes effects of subsequent cancer treatments or palliative care. Given that not all OS and PFS events may occur even during a long follow-up, traditional survival analyses may underestimate survival benefit for tafasitamab + LEN by assuming the same mortality rate for the whole study population.
Aims
To estimate the proportion of long-term survivors (LTS) and survival benefit associated with tafasitamab + LEN treatment using mixture cure models.
Methods
In the L-MIND study (data cut-off: Oct 30, 2020), 80/81 enrolled pts with R/R DLBCL received tafasitamab + LEN, with a median and maximum follow-up duration of 42.7 months (95% confidence interval (CI): 38.0–47.2) and 54.6 months, respectively. Kaplan–Meier (KM) plots of PFS and OS were observed to plateau after 30 months, with the majority of events (PFS: 45/46 [97.8%]; OS: 36/41 [87.8%]) reported up to that time-point. LTS exhibit a high survival benefit from tafasitamab + LEN treatment and were defined as pts without a PFS event and who did not decease after the observed plateau. Thus, the pt population can be considered to consist of two sub-populations: LTS and non-LTS. A ‘mixture cure model’ was fitted on L-MIND observed PFS and OS data to estimate the proportion of LTS and associated mean survival for the two sub-populations. To incorporate background mortality, age- and sex-specific US mortality rates were factored in for the entire study population (enrolled in Europe and US). The weighted average of the mean survival for the two sub-populations provided an estimate of the mean survival for the whole population. For comparative purposes, standard parametric models without considering an LTS proportion were also fitted on PFS and OS data. Sensitivity analyses were performed considering different survival distributions (exponential distribution, Weibull distribution and log-logistic distribution).
Results
Using a mixture cure model fitted to PFS and OS data, the estimated proportion of LTS for the tafasitamab + LEN combination was 41.7% (95% CI: 30.0–54.5) and 44.6% (95% CI: 28.1–62.4), respectively. The predicted mean survival when the mixture cure model was fitted on PFS data was 16.7 years for LTS pts vs 0.5 years for non-LTS pts, yielding 7.4 years for the overall population. Further, survival rates for pts with tafasitamab + LEN treatment were predicted as 40.7% and 36.5% at 2 and 5 years, respectively. Similar results were obtained when the mixture cure model was fitted on OS data. By comparison, using a standard parametric model (Weibull distribution), the predicted mean survival in the overall population was 3.6 years fitting PFS data (Figure 1A) and 5.2 years fitting OS data (Figure 1B).

Conclusion
PFS and OS KM curves for the L-MIND study reaching a long plateau and not following a standard Weibull distribution suggest the presence of an LTS subgroup. Standard parametric models may fail to capture the survival plateau of LTS, leading to a poor estimate of OS benefit in such pts. The mixture cure model suggests that the treatment of R/R DLBCL pts with tafasitamab + LEN is associated with a high LTS proportion of 41.7%.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody


