
Contributions
Abstract: EP549
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Peripheral T-cell lymphoma(PTCL) are characterized by an aggressive clinical behavior. Outcome and treatment options for relapsed or refractory (RR) PTCL patients are poor. Chidamide, an oral novel histone deacetylase inhibitor, has been approved for the treatment of RR PTCL in China.
Aims
We compared the efficacy of chidamide-contained regimens with chemotherapy in RR PTCL patients, in order to identify potentially effective regimens for this cohort.
Methods
We collected the clinical data of ninety-six RR PTCL patients managed in Sun Yat-sen University Cancer Center from January, 2014 to July, 2020. retrospectively. Eligibility criteria included those with R/R disease who had received one prior systemic therapy. Based on the first retreatment regimen, patients were divided into three groups, including chemotherapy(ChT), chemotherapy combined with chidamide(chidamide+ChT) and chidamide combined with or without other targeted agents (targeted therapy) group. Progression-free survival(PFS), overall survival(OS), overall response rate(ORR) and complete response(CR) among three groups were compared. Statistical analysis was performed using IBM SPSS Statistics software (version 24.0). Clinicopathological variables of three treatment groups were assessed by χ2 test or Fisher’ s exact test. Survival curves were plotted using the Kaplan-Meier method and were compared using the log-rank test, with P <0.05 considered to be statistically significant.
Results
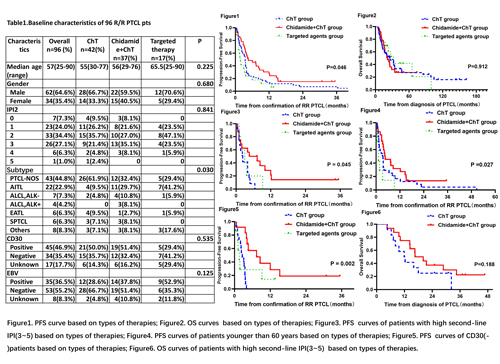
The median follow up time was 20.1 months (range, 4.5-162.4 months). Baseline characteristics of these patients, including the median age, gender, second-line International Prognostic Index (IPI), subtypes of PTCL, CD30 expression and EBV infection, were listed in Table 1. ChT group included more patients with PTCL-NOS, targeted therapy group included more paitents with AITL, other characteristics were well balanced across treatment groups. Chidamide+ ChT group had a significantly better PFS (p=0.046, Figure 1). Median PFS for Chidamide+ChT group was 4.5 months (range 0.4-35.4 months), compared with 3.5 months (range 0.5-65.8 months) for the ChT group and 1.8 months(range 0.4-13.6 months)for the targeted therapy group. No difference in OS was observed(p=0.912, Figure 2). Among patients with high second-line IPI(3-5), there was a significant difference in PFS among three groups(p=0.045, Figure 3). Among patients younger than 60 years, chidamide+ChT group demonstrated a PFS benefit (p =0.027, Figure 4) over other groups. In the subgroup of CD30(-) patients, PFS was superior in the Chidamide+ChT group as compared with other groups (p =0.002, Figure 5). In contrast, there was no statistical difference between the three groups in patients with low second-line IPI (0-2) (p=0.075), or patients older than 60 years (p=0.446) or CD30(+) patients (p =0.575). We found a trend for improved OS for patients with high second-line IPI who received chidamide+ChT as compared with ChT(p=0.188, Figure 6).The ORR for patients in ChT group, chidamide+ChT group and targeted therapy group were 54.8%、64.9% and 64.7%(p=0.607), respectively, with CR rate of 16.7%、18.9% and 35.3%(p=0.262).
Conclusion
Despite this retrospective study with a small sample size, first retreatment with chidamide+ChT regimen showed more PFS benefit than ChT and targeted therapy for RR PTCL patients, especially those with high second-line IPI, younger than 60 years and CD30(-). The combination of chidamide with ChT may be an effective treatment strategy for RR PTCL patients but requires further investigation.
Keyword(s): HDAC inhibitor, Peripheral T-cell lymphoma, Refractory, Relapsed lymphoma
Abstract: EP549
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Peripheral T-cell lymphoma(PTCL) are characterized by an aggressive clinical behavior. Outcome and treatment options for relapsed or refractory (RR) PTCL patients are poor. Chidamide, an oral novel histone deacetylase inhibitor, has been approved for the treatment of RR PTCL in China.
Aims
We compared the efficacy of chidamide-contained regimens with chemotherapy in RR PTCL patients, in order to identify potentially effective regimens for this cohort.
Methods
We collected the clinical data of ninety-six RR PTCL patients managed in Sun Yat-sen University Cancer Center from January, 2014 to July, 2020. retrospectively. Eligibility criteria included those with R/R disease who had received one prior systemic therapy. Based on the first retreatment regimen, patients were divided into three groups, including chemotherapy(ChT), chemotherapy combined with chidamide(chidamide+ChT) and chidamide combined with or without other targeted agents (targeted therapy) group. Progression-free survival(PFS), overall survival(OS), overall response rate(ORR) and complete response(CR) among three groups were compared. Statistical analysis was performed using IBM SPSS Statistics software (version 24.0). Clinicopathological variables of three treatment groups were assessed by χ2 test or Fisher’ s exact test. Survival curves were plotted using the Kaplan-Meier method and were compared using the log-rank test, with P <0.05 considered to be statistically significant.
Results
The median follow up time was 20.1 months (range, 4.5-162.4 months). Baseline characteristics of these patients, including the median age, gender, second-line International Prognostic Index (IPI), subtypes of PTCL, CD30 expression and EBV infection, were listed in Table 1. ChT group included more patients with PTCL-NOS, targeted therapy group included more paitents with AITL, other characteristics were well balanced across treatment groups. Chidamide+ ChT group had a significantly better PFS (p=0.046, Figure 1). Median PFS for Chidamide+ChT group was 4.5 months (range 0.4-35.4 months), compared with 3.5 months (range 0.5-65.8 months) for the ChT group and 1.8 months(range 0.4-13.6 months)for the targeted therapy group. No difference in OS was observed(p=0.912, Figure 2). Among patients with high second-line IPI(3-5), there was a significant difference in PFS among three groups(p=0.045, Figure 3). Among patients younger than 60 years, chidamide+ChT group demonstrated a PFS benefit (p =0.027, Figure 4) over other groups. In the subgroup of CD30(-) patients, PFS was superior in the Chidamide+ChT group as compared with other groups (p =0.002, Figure 5). In contrast, there was no statistical difference between the three groups in patients with low second-line IPI (0-2) (p=0.075), or patients older than 60 years (p=0.446) or CD30(+) patients (p =0.575). We found a trend for improved OS for patients with high second-line IPI who received chidamide+ChT as compared with ChT(p=0.188, Figure 6).The ORR for patients in ChT group, chidamide+ChT group and targeted therapy group were 54.8%、64.9% and 64.7%(p=0.607), respectively, with CR rate of 16.7%、18.9% and 35.3%(p=0.262).

Conclusion
Despite this retrospective study with a small sample size, first retreatment with chidamide+ChT regimen showed more PFS benefit than ChT and targeted therapy for RR PTCL patients, especially those with high second-line IPI, younger than 60 years and CD30(-). The combination of chidamide with ChT may be an effective treatment strategy for RR PTCL patients but requires further investigation.
Keyword(s): HDAC inhibitor, Peripheral T-cell lymphoma, Refractory, Relapsed lymphoma


