
Contributions
Abstract: EP546
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The Burkitt Lymphoma International Prognostic Index Consortium has developed an International Prognostic Index for Burkitt Lymphoma (BL-IPI)(J Clin Oncol 2021: DOI https://doi.org/10.1200/JCO.20). From analysis of a cohort of 633 patients (pts) with BL, four independent variables were identified: age >40 yrs, performance status >2, serum lactate dehydrogenase (LDH) >3 x upper limit normal (ULN), and CNS involvement. This score was externally validated in an independent cohort of 457 patients.
Aims
Our objective was to validate this score in two sequential chemoimmunotherapy trials for patients with BL or leukemia (BLL).
Methods
From 2008 to 2020 two consecutive prospective trials of chemoimmunotherapy for patients with BLL (BURKIMAB-08 and BURKIMAB-14, (Ribera et al Cancer. 2013;119:1660-8, Ribera et al Blood 2019;134 (Suppl 1): 2584) were developed by the Spanish PETHEMA and GELTAMO groups. As the complete response (CR) and overall survival (OS) were similar (CR 85% vs. 80%, 5-yr OS 72% vs. 68%), both trials were merged for validation of the BL-IPI.
Results
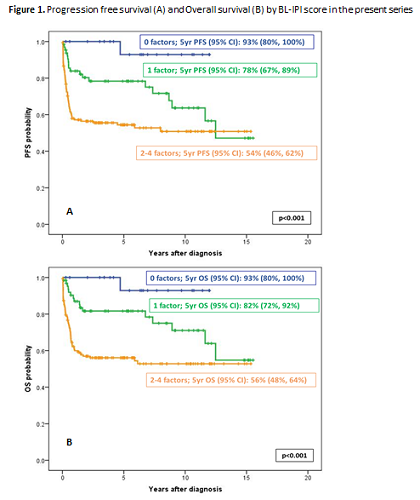
277 pts were included (BURKIMAB-08: 185, BURKIMAB-14: 92), median age (range): 47 (15-83) yrs, Burkitt lymphoma: 193 (70%), Burkitt leukemia: 84 (30%), HIV positive: 74 (27%). Age >40 yrs: 181 (65%), performance status ≥2: 105/272 (39%), LDH ≥3 xULN: 129/220 (59%), CNS involvement: 39/276 (14%). The distribution of patients according to the number of risk factors was: 0: 22 (10%), 1: 65 (28%), 2: 85 (37%), 3: 44 (19%), and 4: 14 (6%). With a median (range) follow-up of 5.4 (0.1-15.5) yrs, the 5-yr progression-free survival (PFS) (±95% CI) for the whole series was 69±6%, and the 5-yr OS (±95% CI) was 71±5%. The 5-yr PFS according to risk groups was: low-risk (0 risk factors; 10% of patients; 93 ±13%), intermediate risk (1 risk factor; 28% of patients; 78±11%), and high-risk (≥2 factors; 62% of patients; 54±8%)(p<0.001, Figure 1A). In turn, the 5-yr OS (±95% CI) according to risk groups was: low-risk (0 risk factors; 10% of patients; 93 ±13%), intermediate risk (1 risk factor; 28% of patients; 82±10%), and high-risk (≥2 factors; 62% of patients; 56±8%)(p<0.001, Figure 1B).
Conclusion
The outcome of our series of pts with BLL was similar to that of BL-IPI series, making feasible the validation. The distribution of patients according to the number of risk factors, as well as the 5-yr PFS and OS according the BL-IPI risk groups were superimposable to that of the BL-IPI. Consequently the BL-IPI was fully validated in our series of pts with BLL.
Supported in part by grant 2017 SGR288 (GRC) Generalitat de Catalunya and “La Caixa” Foundation.
Keyword(s): Burkitt's lymphoma, Prognostic groups
Abstract: EP546
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The Burkitt Lymphoma International Prognostic Index Consortium has developed an International Prognostic Index for Burkitt Lymphoma (BL-IPI)(J Clin Oncol 2021: DOI https://doi.org/10.1200/JCO.20). From analysis of a cohort of 633 patients (pts) with BL, four independent variables were identified: age >40 yrs, performance status >2, serum lactate dehydrogenase (LDH) >3 x upper limit normal (ULN), and CNS involvement. This score was externally validated in an independent cohort of 457 patients.
Aims
Our objective was to validate this score in two sequential chemoimmunotherapy trials for patients with BL or leukemia (BLL).
Methods
From 2008 to 2020 two consecutive prospective trials of chemoimmunotherapy for patients with BLL (BURKIMAB-08 and BURKIMAB-14, (Ribera et al Cancer. 2013;119:1660-8, Ribera et al Blood 2019;134 (Suppl 1): 2584) were developed by the Spanish PETHEMA and GELTAMO groups. As the complete response (CR) and overall survival (OS) were similar (CR 85% vs. 80%, 5-yr OS 72% vs. 68%), both trials were merged for validation of the BL-IPI.
Results
277 pts were included (BURKIMAB-08: 185, BURKIMAB-14: 92), median age (range): 47 (15-83) yrs, Burkitt lymphoma: 193 (70%), Burkitt leukemia: 84 (30%), HIV positive: 74 (27%). Age >40 yrs: 181 (65%), performance status ≥2: 105/272 (39%), LDH ≥3 xULN: 129/220 (59%), CNS involvement: 39/276 (14%). The distribution of patients according to the number of risk factors was: 0: 22 (10%), 1: 65 (28%), 2: 85 (37%), 3: 44 (19%), and 4: 14 (6%). With a median (range) follow-up of 5.4 (0.1-15.5) yrs, the 5-yr progression-free survival (PFS) (±95% CI) for the whole series was 69±6%, and the 5-yr OS (±95% CI) was 71±5%. The 5-yr PFS according to risk groups was: low-risk (0 risk factors; 10% of patients; 93 ±13%), intermediate risk (1 risk factor; 28% of patients; 78±11%), and high-risk (≥2 factors; 62% of patients; 54±8%)(p<0.001, Figure 1A). In turn, the 5-yr OS (±95% CI) according to risk groups was: low-risk (0 risk factors; 10% of patients; 93 ±13%), intermediate risk (1 risk factor; 28% of patients; 82±10%), and high-risk (≥2 factors; 62% of patients; 56±8%)(p<0.001, Figure 1B).

Conclusion
The outcome of our series of pts with BLL was similar to that of BL-IPI series, making feasible the validation. The distribution of patients according to the number of risk factors, as well as the 5-yr PFS and OS according the BL-IPI risk groups were superimposable to that of the BL-IPI. Consequently the BL-IPI was fully validated in our series of pts with BLL.
Supported in part by grant 2017 SGR288 (GRC) Generalitat de Catalunya and “La Caixa” Foundation.
Keyword(s): Burkitt's lymphoma, Prognostic groups


