
Contributions
Abstract: EP540
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Anti-CD19 chimeric antigen receptor cells (CAR T) integrated treatment algorithm for relapsed/refractory large B-cell lymphoma (R/R LBCL). GCSF use is controversial regarding safety and efficacy of CAR T-cells and protection towards infection is not established.
Aims
To evaluate safety, cytopenia, infectious risk and expansion with GCSF in patients (pts) with R/R LBCL treated with Tisagenleuclecel (Tisa-cel) or Axicabtagene-ciloleucel (Axi-cel).
Methods
We included 122 pts treated with CAR T-cells (Axi-cel=63, Tisa-cel=59) between 06/2018 and 11/2020. After 03/2019, GCSF administration from Day (D)5 until ANC >1 G/L showed no impact on CAR T-cells safety and efficacy (1). Hence, after 03/2020 systematic GCSF prophylaxis at D2 was proposed. We identified 3 Groups (G) based on GCSF use: 35 pts in G1 did not receive GCSF during first 10 days, G2 included 33 pts who received systematic D2 GCSF and 54 pts in G3 received GCSF between D5-D10 after CAR T. We analyzed neutropenia duration, infection rate during the first 30 days, cytopenia at month (M)1 and M3, CRS and ICANS incidence and CAR T expansion in the whole population and between groups.
Results
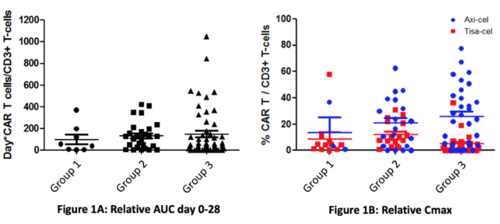
Except for older age (median 52 in G1 vs 65 and 62 in G2 and G3, p=.022) and prior median lines of treatment (G1=3, G2=2, G3=2.5, p<.0001), demographics did not differ in groups. A difference was found for ANC (1570 [0;6540] in G1, 990 [0;4820] in G2 and 920 [0;3630] in G3, p=.007) and lymphocyte count (40 [0;410] in G1, 20 [0;790] in G2 and 30 [0;250] in G3, p=.004) at day 0. Hemoglobin and platelet counts were similar (p=.63 and p=.24 respectively). More pts in G2 had abnormal LDH levels at D0 without significance (p=.059). Median follow-up was 9.6 months after CAR T-cells infusion. Median duration of grade 4 neutropenia was 3 days [0;16] in G1, 4 [0;31] in G2 and 5 [0,63] in G3 (p=.11). 75% of pts experienced febrile neutropenia, with a difference between G1 (74%), G2 (58%) and G3 (85%) (p=.018). 34% of pts in G1, 33% pts in G2 and 26% pts in G3 developed infections in the first month, mostly bacterial (23% in total, 20% in G1, 30% in G2, 20% in G3). Viral infection incidence was 15% in G1, 12% in G2 and 13% in G3 (p=.75). Fungal infections occurred in 3% pts in G1, 3% in G2 and 2% in G3 (p=1). 37% of pts had persistent Grade 3 cytopenia at M1 evaluation, neutropenia being the most frequent (29% vs 8% anemia, vs 28% thrombocytopenia). The same pattern was observed at M3 (21% overall, neutropenia 18% vs 1% anemia, vs 9% thrombocytopenia). CRS occurred in 73% pts overall, mostly of grade 1 (46%) and 2 (27%) without difference on prevalence between the 3 groups (p=.93) nor when considering severe CRS (grade 0-1 vs grade > 2) (p=.28). ICANS, mainly of grade 1 (13%), occurred in 25% patients without difference on prevalence (p=.62) nor on severity (p=.88) when comparing the 3 groups. CAR T-cell expansion did not significantly differ among groups (Fig1A). Tisa-cel and Axi-cel pts were analyzed separately. For Tisa-cel, median relative Cmax CAR T/CD3+ T-cells was 4.3% (median absolute 28 CART/µL) without differences in Gs (median 8.6% and 50/µL in G2 and 3.6% and 13/µL in G3, vs 4.3% and 13/µL in G1)(Fig1B). For Axi-cel, median relative Cmax CAR T/CD3+ T-cells was 21.9% (median absolute 32 CART/µL) without differences according to GCSF use (median 16.5% and 26/µL in G2 and median 24% and 53/µL G3, versus median 3.3% and 12/µL in G1)(Fig1B).
Conclusion
In our experience, early GCSF administration decreases the incidence of febrile neutropenia, with no impact on severity of CRS, ICANS, and CAR T-cells expansion.
Keyword(s): B cell lymphoma, CAR-T, Febrile neutropenia, Granulocyte colony-stimulating factor (G-CSF)
Abstract: EP540
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Anti-CD19 chimeric antigen receptor cells (CAR T) integrated treatment algorithm for relapsed/refractory large B-cell lymphoma (R/R LBCL). GCSF use is controversial regarding safety and efficacy of CAR T-cells and protection towards infection is not established.
Aims
To evaluate safety, cytopenia, infectious risk and expansion with GCSF in patients (pts) with R/R LBCL treated with Tisagenleuclecel (Tisa-cel) or Axicabtagene-ciloleucel (Axi-cel).
Methods
We included 122 pts treated with CAR T-cells (Axi-cel=63, Tisa-cel=59) between 06/2018 and 11/2020. After 03/2019, GCSF administration from Day (D)5 until ANC >1 G/L showed no impact on CAR T-cells safety and efficacy (1). Hence, after 03/2020 systematic GCSF prophylaxis at D2 was proposed. We identified 3 Groups (G) based on GCSF use: 35 pts in G1 did not receive GCSF during first 10 days, G2 included 33 pts who received systematic D2 GCSF and 54 pts in G3 received GCSF between D5-D10 after CAR T. We analyzed neutropenia duration, infection rate during the first 30 days, cytopenia at month (M)1 and M3, CRS and ICANS incidence and CAR T expansion in the whole population and between groups.
Results
Except for older age (median 52 in G1 vs 65 and 62 in G2 and G3, p=.022) and prior median lines of treatment (G1=3, G2=2, G3=2.5, p<.0001), demographics did not differ in groups. A difference was found for ANC (1570 [0;6540] in G1, 990 [0;4820] in G2 and 920 [0;3630] in G3, p=.007) and lymphocyte count (40 [0;410] in G1, 20 [0;790] in G2 and 30 [0;250] in G3, p=.004) at day 0. Hemoglobin and platelet counts were similar (p=.63 and p=.24 respectively). More pts in G2 had abnormal LDH levels at D0 without significance (p=.059). Median follow-up was 9.6 months after CAR T-cells infusion. Median duration of grade 4 neutropenia was 3 days [0;16] in G1, 4 [0;31] in G2 and 5 [0,63] in G3 (p=.11). 75% of pts experienced febrile neutropenia, with a difference between G1 (74%), G2 (58%) and G3 (85%) (p=.018). 34% of pts in G1, 33% pts in G2 and 26% pts in G3 developed infections in the first month, mostly bacterial (23% in total, 20% in G1, 30% in G2, 20% in G3). Viral infection incidence was 15% in G1, 12% in G2 and 13% in G3 (p=.75). Fungal infections occurred in 3% pts in G1, 3% in G2 and 2% in G3 (p=1). 37% of pts had persistent Grade 3 cytopenia at M1 evaluation, neutropenia being the most frequent (29% vs 8% anemia, vs 28% thrombocytopenia). The same pattern was observed at M3 (21% overall, neutropenia 18% vs 1% anemia, vs 9% thrombocytopenia). CRS occurred in 73% pts overall, mostly of grade 1 (46%) and 2 (27%) without difference on prevalence between the 3 groups (p=.93) nor when considering severe CRS (grade 0-1 vs grade > 2) (p=.28). ICANS, mainly of grade 1 (13%), occurred in 25% patients without difference on prevalence (p=.62) nor on severity (p=.88) when comparing the 3 groups. CAR T-cell expansion did not significantly differ among groups (Fig1A). Tisa-cel and Axi-cel pts were analyzed separately. For Tisa-cel, median relative Cmax CAR T/CD3+ T-cells was 4.3% (median absolute 28 CART/µL) without differences in Gs (median 8.6% and 50/µL in G2 and 3.6% and 13/µL in G3, vs 4.3% and 13/µL in G1)(Fig1B). For Axi-cel, median relative Cmax CAR T/CD3+ T-cells was 21.9% (median absolute 32 CART/µL) without differences according to GCSF use (median 16.5% and 26/µL in G2 and median 24% and 53/µL G3, versus median 3.3% and 12/µL in G1)(Fig1B).

Conclusion
In our experience, early GCSF administration decreases the incidence of febrile neutropenia, with no impact on severity of CRS, ICANS, and CAR T-cells expansion.
Keyword(s): B cell lymphoma, CAR-T, Febrile neutropenia, Granulocyte colony-stimulating factor (G-CSF)


