
Contributions
Abstract: EP536
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Diffuse large B-cell lymphoma (DLBCL) exhibits clinical and molecular heterogeneity, and prior studies identified genetic subtypes of DLBCL associated with different trends in response rates and survival. Chimeric antigen receptor T-cell therapy (CAR-T) is approved for the treatment of relapsed/refractory (R/R) DLBCL, but relapses post-CAR-T still occur. Clinical and biological predictors of response to CAR-T remain unclear.
Aims
We investigate the impact of pre-CAR-T next generation sequencing (NGS) results in outcomes of R/R DLBCL post-CAR-T.
Methods
This is a single center experience of R/R DLBCL patients treated with CAR-T between 1/1/2017 and 12/31/2019 who had NGS results via Foundation Medicine (FoM) platform available from prior to CAR-T infusion. The primary objective was to identify predictive mutation profiles with endpoints of overall response rate (ORR), complete response (CR), day 90 CR, and 3-month progression rate. Progression free survival (PFS) probability was calculated with log-rank testing and Kaplan Meier method. Outcomes compared based on NGS results included TP53 mutation (TP53mut) versus wild-type (TP53wt), presence of CDKN2A/B loss, BCL2 rearrangements, and tumor mutation burden (TMB).
Results
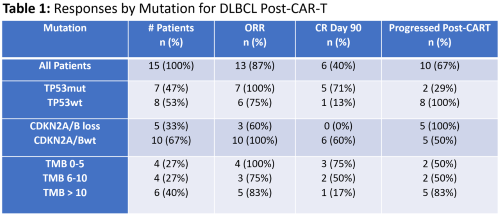
We identified 15 patients with DLBCL who had available NGS results prior to receiving CAR T-cell infusion. Fourteen out of 15 (93%) received axicabtagene ciloleucel, and 1/15 (7%) received tisagenlecleucel. Median age at time of CAR-T was 65 years (range 33-81 years), and 5/15 (33%) were female. FoM specimens were from tumor in 14/15 (93%) and peripheral blood in 1/15 (7%). The most common mutations identified were TP53mut in 7/15 (47%), BCL2 rearrangements in 7/15 (47%), and CDKN2A/B loss in 5/15 (33%). TMB was low (0-5) in 4/15 (27%), intermediate (6-10) in 4/15 (27%), and high (> 10) in 6/15 (40%). One TMB could not be determined.
Outcomes are depicted in Table 1. Best ORR and CR rates were 13/15 (87%) and 8/15 (53%) respectively. ORR (CR) rates were 100% (71%) for TP53mut and 75% (38%) for TP53 wild-type (TP53wt). Day 90 CR rates were 71% for TP53mut and 13% for TP53wt. At last follow-up, 8/8 (100%) TP53wt progressed, but only 2/7 (29%) TP53mut progressed with 5/7 (71%) remaining in CR. The 3-month progression rate was 2/7 (29%) for TP53mut and 6/8 (75%) for TP53wt with significantly higher PFS probability in TP53mut patients (p = 0.002). The 3-month progression rates for CDKN2A/B loss and wild-type were 5/5 (100%) and 3/10 (30%) respectively with lower PFS probability in patients with CDKN2A/B loss (p<0.001). There was no significant difference in PFS based on BCL2 rearrangement or TMB.
There was no significant difference in patients’ age, gender, lactate dehydrogenase, international prognostic index score, extranodal disease, stage, number of prior lines of therapy, history of autologous stem cell transplant, or frequency of bridging therapy between TP53, CDKN2A/B, and TMB groups.
Conclusion
In this small dataset, our findings show a potential prognostic role of NGS in R/R DLBCL treated with CAR-T. Significant differences were seen with better outcomes post-CAR-T associated with the presence of TP53 mutations and worse outcomes with CDKN2A/B loss. Improved responses with TP53mut may be related to higher T-cell infiltration, tumor inflammation signature, expression of immune checkpoints, and tumor microenvironment changes that have been reported in TP53mut acute myeloid leukemia (Vadakekolathu J et al. Blood Adv 2020). Larger studies are needed to further characterize the impact of NGS and molecular changes on outcomes in DLBCL post-CAR-T.
Keyword(s): CAR-T, Diffuse large B cell lymphoma, Mutation, P53
Abstract: EP536
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Diffuse large B-cell lymphoma (DLBCL) exhibits clinical and molecular heterogeneity, and prior studies identified genetic subtypes of DLBCL associated with different trends in response rates and survival. Chimeric antigen receptor T-cell therapy (CAR-T) is approved for the treatment of relapsed/refractory (R/R) DLBCL, but relapses post-CAR-T still occur. Clinical and biological predictors of response to CAR-T remain unclear.
Aims
We investigate the impact of pre-CAR-T next generation sequencing (NGS) results in outcomes of R/R DLBCL post-CAR-T.
Methods
This is a single center experience of R/R DLBCL patients treated with CAR-T between 1/1/2017 and 12/31/2019 who had NGS results via Foundation Medicine (FoM) platform available from prior to CAR-T infusion. The primary objective was to identify predictive mutation profiles with endpoints of overall response rate (ORR), complete response (CR), day 90 CR, and 3-month progression rate. Progression free survival (PFS) probability was calculated with log-rank testing and Kaplan Meier method. Outcomes compared based on NGS results included TP53 mutation (TP53mut) versus wild-type (TP53wt), presence of CDKN2A/B loss, BCL2 rearrangements, and tumor mutation burden (TMB).
Results
We identified 15 patients with DLBCL who had available NGS results prior to receiving CAR T-cell infusion. Fourteen out of 15 (93%) received axicabtagene ciloleucel, and 1/15 (7%) received tisagenlecleucel. Median age at time of CAR-T was 65 years (range 33-81 years), and 5/15 (33%) were female. FoM specimens were from tumor in 14/15 (93%) and peripheral blood in 1/15 (7%). The most common mutations identified were TP53mut in 7/15 (47%), BCL2 rearrangements in 7/15 (47%), and CDKN2A/B loss in 5/15 (33%). TMB was low (0-5) in 4/15 (27%), intermediate (6-10) in 4/15 (27%), and high (> 10) in 6/15 (40%). One TMB could not be determined.
Outcomes are depicted in Table 1. Best ORR and CR rates were 13/15 (87%) and 8/15 (53%) respectively. ORR (CR) rates were 100% (71%) for TP53mut and 75% (38%) for TP53 wild-type (TP53wt). Day 90 CR rates were 71% for TP53mut and 13% for TP53wt. At last follow-up, 8/8 (100%) TP53wt progressed, but only 2/7 (29%) TP53mut progressed with 5/7 (71%) remaining in CR. The 3-month progression rate was 2/7 (29%) for TP53mut and 6/8 (75%) for TP53wt with significantly higher PFS probability in TP53mut patients (p = 0.002). The 3-month progression rates for CDKN2A/B loss and wild-type were 5/5 (100%) and 3/10 (30%) respectively with lower PFS probability in patients with CDKN2A/B loss (p<0.001). There was no significant difference in PFS based on BCL2 rearrangement or TMB.
There was no significant difference in patients’ age, gender, lactate dehydrogenase, international prognostic index score, extranodal disease, stage, number of prior lines of therapy, history of autologous stem cell transplant, or frequency of bridging therapy between TP53, CDKN2A/B, and TMB groups.

Conclusion
In this small dataset, our findings show a potential prognostic role of NGS in R/R DLBCL treated with CAR-T. Significant differences were seen with better outcomes post-CAR-T associated with the presence of TP53 mutations and worse outcomes with CDKN2A/B loss. Improved responses with TP53mut may be related to higher T-cell infiltration, tumor inflammation signature, expression of immune checkpoints, and tumor microenvironment changes that have been reported in TP53mut acute myeloid leukemia (Vadakekolathu J et al. Blood Adv 2020). Larger studies are needed to further characterize the impact of NGS and molecular changes on outcomes in DLBCL post-CAR-T.
Keyword(s): CAR-T, Diffuse large B cell lymphoma, Mutation, P53


