
Contributions
Abstract: EP535
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Bispecific anti-CD20, anti-CD19 (LV20.19) CAR T-cells may improve outcomes in relapsed, refractory (R/R) B-cell non-Hodgkin lymphoma (NHL) by limiting relapse due to single antigen downregulation. We reported outcomes of a phase I trial of LV20.19 CAR T-cells in R/R NHL & CLL (Shah et al. Nature Med. 2020). There is evidence that higher LDH and baseline tumor burden (as measured by total lesion glycolysis [TLG] and metabolic tumor volume [MTV]) predict inferior response and increased toxicities for single antigen targeted anti-CD19 CAR-T cells. In the context of bispecific CAR T-cells, the impact of tumor burden on outcomes is not known.
Aims
We analyzed whether baseline LDH and tumor burden assessed by whole-body TLG correlated with response and/or toxicities for bispecific CAR T-cells.
Methods
To evaluate the relationship of baseline LDH and tumor burden on day 28 response and toxicities (cytokine release syndrome [CRS] and neurotoxicity), we included patients from the previously mentioned Phase 1 clinical trial treated with LV20.19 CAR T-cells (NCT03019055) who had a pre-CAR-T PET/CT within 45 days of lymphodepleting chemotherapy (LDC). Patients with CLL were excluded. Baseline tumor burden was assessed by measurement of whole body TLG on most recent pre-CAR-T PET/CT within 45 days of LDC. LDH was assessed on day 0 of LDC. Threshold values for high and low tumor volume groups were selected based on the median TLG as previously reported. Continuous and categorical variables were analyzed using t-test, Pearson's chi-squared test and Fischer's exact test where appropriate. All tests were two sided. A p value <0.05 was considered significant.
Results
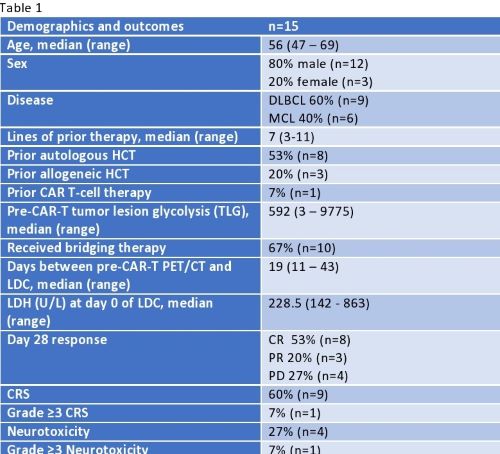
15 patients were included in the analysis (9 DLBCL, 6 MCL). Patient characteristics are summarized in table 1. Median age was 56, median lines of prior therapy was 7 (range 3-11) and 67% received bridging therapy after apheresis. Median baseline LDH was 228.5 U/L. Median TLG was 592 (range 3-9775) and served as the threshold value for low and high tumor burden groups (≤592 and >592 respectively). On day 28, 53% (n=8) had a complete response (CR), 20% (n=3) a partial response (PR), and 27% (n=4) had progressive disease (PD). CRS occurred in 60% and neurotoxicity in 27% of patients. Mean baseline LDH was significantly lower in patients who had a response (CR or PR) (243 vs 600, p=0.03) and in those with CR (196 vs 504.2, p=0.046). Low tumor burden on PET/CT was associated with overall response (CR or PR) vs PD (p=0.026) but not with a CR (p=0.13). High tumor burden was not associated with the development of any grade CRS (p=0.83) or neurotoxicity (p=0.18).
Conclusion
This is the first study demonstrating that baseline tumor burden (by TLG on PET/CT) correlates with clinical outcomes in the context of bispecific CAR T-cells for NHL. Moreover, both patients with CR and CR+PR had a significantly lower baseline LDH than patients who had PD +/- PR on day 28 scans. Interestingly, baseline tumor burden did not correlate with development of CRS or neurotoxicity with bispecific LV20.19 CAR T-cells. In single targeted CD19 CAR T-cells, high baseline tumor burden has previously been correlated with toxicity as well as lower response rates. In our LV20.19 experience, overall CRS and neurotoxicity rates were low and the low overall incidence may limit statistical power to detect an association with tumor burden. With accumulating evidence that higher pre–CAR-T tumor burden is associated with inferior response rates, developing novel approaches to debulk patients safely prior to CAR-T is indicated.
Keyword(s):
Abstract: EP535
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Bispecific anti-CD20, anti-CD19 (LV20.19) CAR T-cells may improve outcomes in relapsed, refractory (R/R) B-cell non-Hodgkin lymphoma (NHL) by limiting relapse due to single antigen downregulation. We reported outcomes of a phase I trial of LV20.19 CAR T-cells in R/R NHL & CLL (Shah et al. Nature Med. 2020). There is evidence that higher LDH and baseline tumor burden (as measured by total lesion glycolysis [TLG] and metabolic tumor volume [MTV]) predict inferior response and increased toxicities for single antigen targeted anti-CD19 CAR-T cells. In the context of bispecific CAR T-cells, the impact of tumor burden on outcomes is not known.
Aims
We analyzed whether baseline LDH and tumor burden assessed by whole-body TLG correlated with response and/or toxicities for bispecific CAR T-cells.
Methods
To evaluate the relationship of baseline LDH and tumor burden on day 28 response and toxicities (cytokine release syndrome [CRS] and neurotoxicity), we included patients from the previously mentioned Phase 1 clinical trial treated with LV20.19 CAR T-cells (NCT03019055) who had a pre-CAR-T PET/CT within 45 days of lymphodepleting chemotherapy (LDC). Patients with CLL were excluded. Baseline tumor burden was assessed by measurement of whole body TLG on most recent pre-CAR-T PET/CT within 45 days of LDC. LDH was assessed on day 0 of LDC. Threshold values for high and low tumor volume groups were selected based on the median TLG as previously reported. Continuous and categorical variables were analyzed using t-test, Pearson's chi-squared test and Fischer's exact test where appropriate. All tests were two sided. A p value <0.05 was considered significant.
Results
15 patients were included in the analysis (9 DLBCL, 6 MCL). Patient characteristics are summarized in table 1. Median age was 56, median lines of prior therapy was 7 (range 3-11) and 67% received bridging therapy after apheresis. Median baseline LDH was 228.5 U/L. Median TLG was 592 (range 3-9775) and served as the threshold value for low and high tumor burden groups (≤592 and >592 respectively). On day 28, 53% (n=8) had a complete response (CR), 20% (n=3) a partial response (PR), and 27% (n=4) had progressive disease (PD). CRS occurred in 60% and neurotoxicity in 27% of patients. Mean baseline LDH was significantly lower in patients who had a response (CR or PR) (243 vs 600, p=0.03) and in those with CR (196 vs 504.2, p=0.046). Low tumor burden on PET/CT was associated with overall response (CR or PR) vs PD (p=0.026) but not with a CR (p=0.13). High tumor burden was not associated with the development of any grade CRS (p=0.83) or neurotoxicity (p=0.18).

Conclusion
This is the first study demonstrating that baseline tumor burden (by TLG on PET/CT) correlates with clinical outcomes in the context of bispecific CAR T-cells for NHL. Moreover, both patients with CR and CR+PR had a significantly lower baseline LDH than patients who had PD +/- PR on day 28 scans. Interestingly, baseline tumor burden did not correlate with development of CRS or neurotoxicity with bispecific LV20.19 CAR T-cells. In single targeted CD19 CAR T-cells, high baseline tumor burden has previously been correlated with toxicity as well as lower response rates. In our LV20.19 experience, overall CRS and neurotoxicity rates were low and the low overall incidence may limit statistical power to detect an association with tumor burden. With accumulating evidence that higher pre–CAR-T tumor burden is associated with inferior response rates, developing novel approaches to debulk patients safely prior to CAR-T is indicated.
Keyword(s):


