
Contributions
Abstract: EP534
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Primary vitreoretinal lymphoma (PVRL) is a rare variant of primary central nervous system (CNS) lymphoma, for which currently there are no optimal treatment options.
Aims
To balance the adverse events (AEs) of chemotherapy and achieve sustained control of lymphoma, we conducted a single-arm, phase II trial for newly diagnosed B-cell PVRL to evaluate the efficacy and safety of the R2 regimen with intravitreal MTX
Methods
This prospective single-center study enrolled immunocompetent patients with newly diagnosed PVRL between August 2018 and January 2020. Patients received local and systemic therapies: intravitreal methotrexate (MTX, 400 μg, 0.1 mL) injections for 1 year (total 16 injections) and six cycles of the rituximab (375 mg/m2 on day 1) and lenalidomide (25 mg on day 1–21; R2) regimen. Lenalidomide was maintained for 2 years in patients who had achieved a response. This trial is registered with ClinicalTrials.gov (number NCT 03746223).
Results
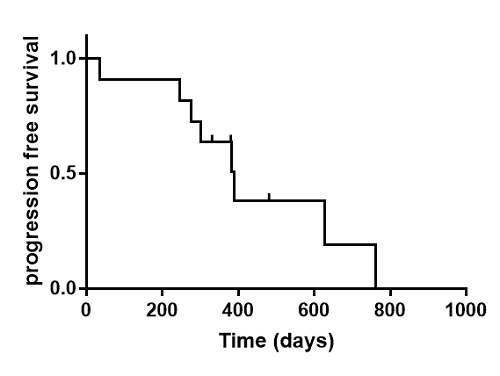
We enrolled 11 patients with a mean age of 58 (range, 48–70) years, of which 10 achieved complete remission at the first evaluation. The median follow-up period was 18.3 (range, 10.6–27.8) months, and the median progression-free survival was 12.7 months. Moreover, a total of eight patients relapsed. The most common adverse event (AE) was neutropenia, which occurred in seven patients (63.6%), followed by grade 3 ocular toxicities, including cataract formation, in six patients (54%).
Conclusion
These findings suggest that the R2 regimen combined with intravitreal MTX, followed by lenalidomide maintenance, is a safe option for PVRL with moderate efficacy.
Keyword(s): CNS lymphoma, Imids, Lymphoma therapy, Phase II
Abstract: EP534
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Primary vitreoretinal lymphoma (PVRL) is a rare variant of primary central nervous system (CNS) lymphoma, for which currently there are no optimal treatment options.
Aims
To balance the adverse events (AEs) of chemotherapy and achieve sustained control of lymphoma, we conducted a single-arm, phase II trial for newly diagnosed B-cell PVRL to evaluate the efficacy and safety of the R2 regimen with intravitreal MTX
Methods
This prospective single-center study enrolled immunocompetent patients with newly diagnosed PVRL between August 2018 and January 2020. Patients received local and systemic therapies: intravitreal methotrexate (MTX, 400 μg, 0.1 mL) injections for 1 year (total 16 injections) and six cycles of the rituximab (375 mg/m2 on day 1) and lenalidomide (25 mg on day 1–21; R2) regimen. Lenalidomide was maintained for 2 years in patients who had achieved a response. This trial is registered with ClinicalTrials.gov (number NCT 03746223).
Results
We enrolled 11 patients with a mean age of 58 (range, 48–70) years, of which 10 achieved complete remission at the first evaluation. The median follow-up period was 18.3 (range, 10.6–27.8) months, and the median progression-free survival was 12.7 months. Moreover, a total of eight patients relapsed. The most common adverse event (AE) was neutropenia, which occurred in seven patients (63.6%), followed by grade 3 ocular toxicities, including cataract formation, in six patients (54%).

Conclusion
These findings suggest that the R2 regimen combined with intravitreal MTX, followed by lenalidomide maintenance, is a safe option for PVRL with moderate efficacy.
Keyword(s): CNS lymphoma, Imids, Lymphoma therapy, Phase II


