
Contributions
Abstract: EP532
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Systemic anaplastic large cell lymphoma (sALCL) is a T-cell lymphoma, historically associated with low cure rates following relapse. Expression of the ALK protein is more common in younger patients and associated with improved survival outcomes. Following a pivotal phase II study (Pro et al) and European licence, the immuno-conjugate Brentuximab Vedotin (BV) became available in England via the Cancer Drugs Fund (CDF) in 2013. To date, no large-scale population-based studies have described outcomes following BV. Public Health England (PHE) collects information on all cancer diagnoses in England, and since April 2012 all delivered Systemic Anti-Cancer Therapy (SACT).
Aims
We aimed to evaluate whether routinely collected PHE data can reliably assess outcomes of patients with lymphoma. Survival outcomes for BV monotherapy in relapsed ALCL in all England was chosen as a pilot project to explore the value of PHE data due to the well-defined disease and SACT cohort.
Methods
Following NHS REC approval, we requested anonymised Office of Data Release (ODR) information on sALCL patients ≥18 years treated with BV monotherapy between 1st Jan 2014-31st Dec 2019. We excluded primary cutaneous and breast-implant associated ALCL, patients in the ECHELON-2 trial and BV received in combination. We requested baseline demographics, ALK status, dates and number of BV cycles, and SACT data prior to and following BV, prior autologous/allogeneic stem cell transplantation (SCT). Kaplan-Meier survival methods were used to compare the primary outcome of all-cause mortality between groups defined on the basis of age, gender and ALK status (positive vs. negative) in univariate analyses.
Results
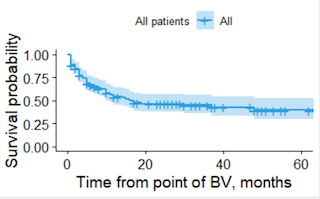
173 patients were identified, of whom 46 were excluded. The final cohort comprised 127 patients with r/r ALCL with a median age of 60 years at initiation of BV and median follow up time of 10 months. 49 (38.6%) patients were ALK+ve and 78 (61.4%) ALK-ve. The median time from diagnosis to BV was nine months (range 1-143). 106 (83.5%) received BV second line, and 13 (10.2%) third or fourth line. 95 (74.8%) received CHOP first line. Based on SACT data 18 (14.2%) received SCT in first remission (16 auto, 2 allo). The median number of BV cycles received was five. Median 2-year overall survival (OS) was 46.6%. The majority (59) of deaths occurred within the first 18 months, followed by a survival plateau with only four subsequent deaths. There were 20 (15.7%) deaths prior to cycle 5, the timepoint where the CDF mandates response assessment. There was no difference in OS between ALK+ve and ALK-ve (p=0.78), age <40 vs ≥40 years(p=0.89), age <60 vs ≥60(p=0.96), between genders(p=0.25), or receiving prior SCT (p=0.55). Two patients received SCT after BV, with both alive 20+ months post-transplant. Receiving BV second line therapy was associated with improved survival, with 2-year OS of 50.3% versus 29.7% for those third or fourth line(p = 0.03).
Conclusion
We confirm BV is a highly effective treatment for r/r sALCL with real world survival outcomes across sub-groups highly comparable with clinical trial data. Weaknesses of this study include lack of information on toxicity and data from double blind studies, although this data is now being collected. Analysis of routinely collected PHE data offers the opportunity to undertake high quality, nationwide population-based studies, to inform the efficacy of high-cost drugs such as BV in routine clinical practice.
Keyword(s): ALCL, Survival, T cell lymphoma
Abstract: EP532
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Systemic anaplastic large cell lymphoma (sALCL) is a T-cell lymphoma, historically associated with low cure rates following relapse. Expression of the ALK protein is more common in younger patients and associated with improved survival outcomes. Following a pivotal phase II study (Pro et al) and European licence, the immuno-conjugate Brentuximab Vedotin (BV) became available in England via the Cancer Drugs Fund (CDF) in 2013. To date, no large-scale population-based studies have described outcomes following BV. Public Health England (PHE) collects information on all cancer diagnoses in England, and since April 2012 all delivered Systemic Anti-Cancer Therapy (SACT).
Aims
We aimed to evaluate whether routinely collected PHE data can reliably assess outcomes of patients with lymphoma. Survival outcomes for BV monotherapy in relapsed ALCL in all England was chosen as a pilot project to explore the value of PHE data due to the well-defined disease and SACT cohort.
Methods
Following NHS REC approval, we requested anonymised Office of Data Release (ODR) information on sALCL patients ≥18 years treated with BV monotherapy between 1st Jan 2014-31st Dec 2019. We excluded primary cutaneous and breast-implant associated ALCL, patients in the ECHELON-2 trial and BV received in combination. We requested baseline demographics, ALK status, dates and number of BV cycles, and SACT data prior to and following BV, prior autologous/allogeneic stem cell transplantation (SCT). Kaplan-Meier survival methods were used to compare the primary outcome of all-cause mortality between groups defined on the basis of age, gender and ALK status (positive vs. negative) in univariate analyses.
Results
173 patients were identified, of whom 46 were excluded. The final cohort comprised 127 patients with r/r ALCL with a median age of 60 years at initiation of BV and median follow up time of 10 months. 49 (38.6%) patients were ALK+ve and 78 (61.4%) ALK-ve. The median time from diagnosis to BV was nine months (range 1-143). 106 (83.5%) received BV second line, and 13 (10.2%) third or fourth line. 95 (74.8%) received CHOP first line. Based on SACT data 18 (14.2%) received SCT in first remission (16 auto, 2 allo). The median number of BV cycles received was five. Median 2-year overall survival (OS) was 46.6%. The majority (59) of deaths occurred within the first 18 months, followed by a survival plateau with only four subsequent deaths. There were 20 (15.7%) deaths prior to cycle 5, the timepoint where the CDF mandates response assessment. There was no difference in OS between ALK+ve and ALK-ve (p=0.78), age <40 vs ≥40 years(p=0.89), age <60 vs ≥60(p=0.96), between genders(p=0.25), or receiving prior SCT (p=0.55). Two patients received SCT after BV, with both alive 20+ months post-transplant. Receiving BV second line therapy was associated with improved survival, with 2-year OS of 50.3% versus 29.7% for those third or fourth line(p = 0.03).

Conclusion
We confirm BV is a highly effective treatment for r/r sALCL with real world survival outcomes across sub-groups highly comparable with clinical trial data. Weaknesses of this study include lack of information on toxicity and data from double blind studies, although this data is now being collected. Analysis of routinely collected PHE data offers the opportunity to undertake high quality, nationwide population-based studies, to inform the efficacy of high-cost drugs such as BV in routine clinical practice.
Keyword(s): ALCL, Survival, T cell lymphoma


