
Contributions
Abstract: EP529
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The rituximab biosimilar CT-P10 was granted European Medicines Agency approval in 2017 for the treatment of rheumatoid arthritis (RA) and specific blood cancers, including non-Hodgkin’s lymphoma (NHL). Diffuse large B-cell lymphoma (DLBCL) accounts for 30–50% of NHL cases. Clinical similarity between CT-P10 and rituximab was accepted based on evidence in populations of patients with RA and follicular lymphoma. To-date, DLBCL patients have not been included in clinical trials of CT-P10 and no studies have investigated the effectiveness or safety of CT-P10 treatment in patients with DLBCL in a European real world clinical setting.
Aims
To evaluate the real world clinical effectiveness and safety of CT-P10 in patients with DLBCL in Europe.
Methods
This non-interventional post-authorisation safety study involved collection of patient-level data from hospital medical records for patients with DLBCL who received CT-P10 treatment prescribed in accordance with the marketing authorisation in five European countries (United Kingdom, Spain, France, Germany and Italy). Patients were treated as per routine clinical practice. Effectiveness (overall survival [OS] and progression free survival [PFS], CT-P10 responses), safety (adverse events [AEs] and infusion-related reactions [IRRs]) and CT-P10 treatment pattern data were collected during the 30-month observation period following the index date (date of CT-P10 initiation) or until death, if sooner.
Results
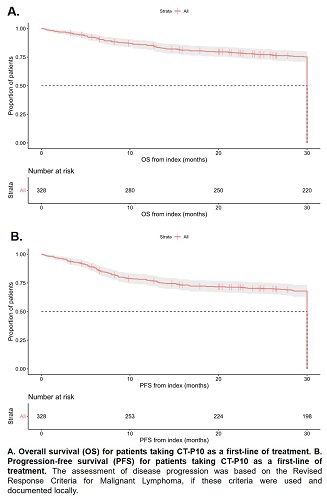
This study included 389 (58% male) patients with a median disease duration at index of 23.0 (Interquartile range [IQR] 12.0–46.0) days. The median age at index was 69.7 (IQR 60.3–76.1) years. Of patients with available data at index, 42% (n=98/191) had Ann Arbor stage IV disease while 21% (n=49/237) had an Eastern Cooperative Oncology Group performance score ≥2; 50% (n=131/263) of patients were classified as intermediate-high or high risk (International Prognostic Index score ≥3). CT-P10 was received as a first-line treatment for DLBCL by 84% (n=328) of patients, second-line for 11% (n=41), and third-line or greater for 5% (n=20). R-CHOP was the most administered chemotherapy regimen at index (68%, n=266/389). The best recorded response to CT-P10 (n=382) was complete response in 82% (n=312) of patients, partial response in 12% (n=46), no response or stable disease in 4% (n=16) and progressive disease in 2% (n=8). At 30-months post-index, for patients taking first-line CT-P10, 74% (95% confidence interval [CI] 69.2–79.1%) of patients were still alive and 67% (95%CI 61.3–72.1%) had not progressed. Kaplan-Meier curves depicting OS and PFS are shown in Figure 1; median OS/PFS was not reached. Overall, 90% (n=351) of patients reported at least one AE (n=2504 total AEs); 46% (n=178) had ≥1 Grade 3 AEs, most commonly neutropenia (9% [n=35/369] of all Grade 3 AEs). 9% (n=180/2100) of AEs were recorded as definitely, probably or possibly related to CT-P10 and 6% (n=24) of patients discontinued CT-P10 due to AEs. IRRs were reported on day 1 or 2 for 8% (n=31) of patients.
Conclusion
This study provides new insight into the real world effectiveness and safety of CT-P10 for the treatment of DLBCL. Over three quarters of patients initiated on CT-P10 achieved complete or partial response by 30 months, with CT-P10 appearing to be generally well tolerated by patients. The response and survival rates and overall safety profile for CT-P10 appear consistent with those reported for reference rituximab, supporting CT-P10 use in combination with chemotherapy as a therapeutic option for DLBCL.
Keyword(s): DLBCL, Non-Hodgkin's lymphoma, Rituximab, Safety
Abstract: EP529
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The rituximab biosimilar CT-P10 was granted European Medicines Agency approval in 2017 for the treatment of rheumatoid arthritis (RA) and specific blood cancers, including non-Hodgkin’s lymphoma (NHL). Diffuse large B-cell lymphoma (DLBCL) accounts for 30–50% of NHL cases. Clinical similarity between CT-P10 and rituximab was accepted based on evidence in populations of patients with RA and follicular lymphoma. To-date, DLBCL patients have not been included in clinical trials of CT-P10 and no studies have investigated the effectiveness or safety of CT-P10 treatment in patients with DLBCL in a European real world clinical setting.
Aims
To evaluate the real world clinical effectiveness and safety of CT-P10 in patients with DLBCL in Europe.
Methods
This non-interventional post-authorisation safety study involved collection of patient-level data from hospital medical records for patients with DLBCL who received CT-P10 treatment prescribed in accordance with the marketing authorisation in five European countries (United Kingdom, Spain, France, Germany and Italy). Patients were treated as per routine clinical practice. Effectiveness (overall survival [OS] and progression free survival [PFS], CT-P10 responses), safety (adverse events [AEs] and infusion-related reactions [IRRs]) and CT-P10 treatment pattern data were collected during the 30-month observation period following the index date (date of CT-P10 initiation) or until death, if sooner.
Results
This study included 389 (58% male) patients with a median disease duration at index of 23.0 (Interquartile range [IQR] 12.0–46.0) days. The median age at index was 69.7 (IQR 60.3–76.1) years. Of patients with available data at index, 42% (n=98/191) had Ann Arbor stage IV disease while 21% (n=49/237) had an Eastern Cooperative Oncology Group performance score ≥2; 50% (n=131/263) of patients were classified as intermediate-high or high risk (International Prognostic Index score ≥3). CT-P10 was received as a first-line treatment for DLBCL by 84% (n=328) of patients, second-line for 11% (n=41), and third-line or greater for 5% (n=20). R-CHOP was the most administered chemotherapy regimen at index (68%, n=266/389). The best recorded response to CT-P10 (n=382) was complete response in 82% (n=312) of patients, partial response in 12% (n=46), no response or stable disease in 4% (n=16) and progressive disease in 2% (n=8). At 30-months post-index, for patients taking first-line CT-P10, 74% (95% confidence interval [CI] 69.2–79.1%) of patients were still alive and 67% (95%CI 61.3–72.1%) had not progressed. Kaplan-Meier curves depicting OS and PFS are shown in Figure 1; median OS/PFS was not reached. Overall, 90% (n=351) of patients reported at least one AE (n=2504 total AEs); 46% (n=178) had ≥1 Grade 3 AEs, most commonly neutropenia (9% [n=35/369] of all Grade 3 AEs). 9% (n=180/2100) of AEs were recorded as definitely, probably or possibly related to CT-P10 and 6% (n=24) of patients discontinued CT-P10 due to AEs. IRRs were reported on day 1 or 2 for 8% (n=31) of patients.

Conclusion
This study provides new insight into the real world effectiveness and safety of CT-P10 for the treatment of DLBCL. Over three quarters of patients initiated on CT-P10 achieved complete or partial response by 30 months, with CT-P10 appearing to be generally well tolerated by patients. The response and survival rates and overall safety profile for CT-P10 appear consistent with those reported for reference rituximab, supporting CT-P10 use in combination with chemotherapy as a therapeutic option for DLBCL.
Keyword(s): DLBCL, Non-Hodgkin's lymphoma, Rituximab, Safety


