
Contributions
Abstract: EP528
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Recent years have seen the emergence of novel treatments for Relapse/Refractory diffuse large B-cell lymphoma (DLBCL). There remains a paucity, however, of published analyses examining outcomes of patients(pts) within the evolving treatment landscape.
Aims
To address this data gap, this study examined real-world clinical outcomes among pts with R/R DLBCL who received third or subsequent line (≥3L) of therapy.
Methods
This retrospective analysis included adult pts, diagnosed with DLBCL [01/01/2014 to 31/08/2019], who received ≥3L of therapy. Data were abstracted from COTA, a de-identified database of real-world data, derived from the electronic health records of healthcare providers in the United States. Pts were categorized as receiving 1) chemotherapy or chemoimmunotherapy (CT/CIT), 2) targeted therapy (TT) [brentuximab, vedotin, ibrutinib, venetoclax, lenalidomide, obinutuzumab, nivolumab, polatuzumab, and pembrolizumab], 3) chimeric antigen receptor T cells (CAR-T), or 4) salvage therapy consolidated with hematopoietic stem cell transplantation (HSCT). Patient characteristics, response rates, and overall survival (OS) were reported by line of therapy (3L, 4L) for the overall study population and stratified by treatment category. HSCT being a consolidation therapy for pts achieving a complete or partial remission (CR or PR) following salvage treatment, hence; response rates following HSCT were not reported.
Results
Of the 435 pts identified, 212 received 3L and 128 received 4L. At 3L initiation, pts had a mean age of 61yrs (range 18-90) and 59% were male. At 4L initiation, pts had a mean age of 60yrs (range 20-84) and 61% were male. Within a median follow-up of 22.5 months, 14.9% of pts treated with 3L CT/CIT or TT received subsequent CAR-T. Within a median follow-up of 19.3 months, 10.9% of pts treated with 4L CT/CIT or TT received subsequent CAR-T.
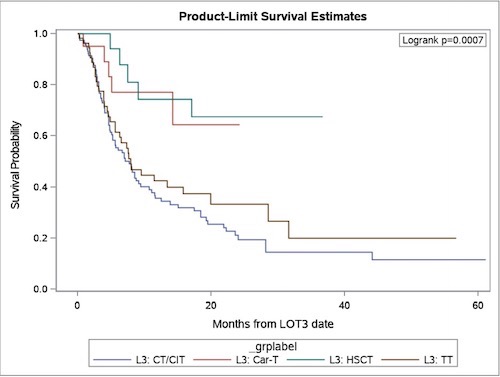
CR rates of pts receiving non-cell therapy were substantially lower than pts receiving CAR-T, in both 3L and 4L settings [Table1]. OS results for 3L therapy are presented in Figure 1. Median OS of non-cell therapy was 7.7 months (CT/CIT: 7.7 months; TT: 7.9 months), and 4.5 months (CT/CIT: 4.5 months; TT: 5.1 months) for 3L and 4L, respectively. Median survival time of pts receiving cell therapy (CAR-T and HSCT) had not reached in either the 3L or 4L setting.
Table 1: Treatment patterns and complete response rates for 3rd Line and 4th Line pts
| 3L | 4L | ||
n (%) | CR rate | n (%) | CR rate | |
CT/CIT | 117 (55) | 9.4% | 66 (52) | 7.6% |
TT | 57 (27) | 8.8% | 44 (34) | 15.9% |
CAR-T | 20 (9) | 60% | 12 (9) | 50% |
HSCT | 18 (8) | N/A | 6 (5) | N/A |
Conclusion
Results from this real-world study illustrate that majority of pts treated with 3L CT/CIT or TT, had poor clinical outcomes, which were exacerbated in the 4L setting. Pts who received cell therapy had better clinical outcomes, however, the proportion of pts receiving these treatments is low. Preliminary data suggests that survival outcomes among CAR-T and HSCT pts, are similar in the 3L and 4L settings. Despite various novel treatments, prognosis for pts with R/R DLBCL remains very poor, underscoring the need for more efficacious treatment options.
Keyword(s): DLBCL, Relapsed lymphoma, Survival, Treatment
Abstract: EP528
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Recent years have seen the emergence of novel treatments for Relapse/Refractory diffuse large B-cell lymphoma (DLBCL). There remains a paucity, however, of published analyses examining outcomes of patients(pts) within the evolving treatment landscape.
Aims
To address this data gap, this study examined real-world clinical outcomes among pts with R/R DLBCL who received third or subsequent line (≥3L) of therapy.
Methods
This retrospective analysis included adult pts, diagnosed with DLBCL [01/01/2014 to 31/08/2019], who received ≥3L of therapy. Data were abstracted from COTA, a de-identified database of real-world data, derived from the electronic health records of healthcare providers in the United States. Pts were categorized as receiving 1) chemotherapy or chemoimmunotherapy (CT/CIT), 2) targeted therapy (TT) [brentuximab, vedotin, ibrutinib, venetoclax, lenalidomide, obinutuzumab, nivolumab, polatuzumab, and pembrolizumab], 3) chimeric antigen receptor T cells (CAR-T), or 4) salvage therapy consolidated with hematopoietic stem cell transplantation (HSCT). Patient characteristics, response rates, and overall survival (OS) were reported by line of therapy (3L, 4L) for the overall study population and stratified by treatment category. HSCT being a consolidation therapy for pts achieving a complete or partial remission (CR or PR) following salvage treatment, hence; response rates following HSCT were not reported.
Results
Of the 435 pts identified, 212 received 3L and 128 received 4L. At 3L initiation, pts had a mean age of 61yrs (range 18-90) and 59% were male. At 4L initiation, pts had a mean age of 60yrs (range 20-84) and 61% were male. Within a median follow-up of 22.5 months, 14.9% of pts treated with 3L CT/CIT or TT received subsequent CAR-T. Within a median follow-up of 19.3 months, 10.9% of pts treated with 4L CT/CIT or TT received subsequent CAR-T.
CR rates of pts receiving non-cell therapy were substantially lower than pts receiving CAR-T, in both 3L and 4L settings [Table1]. OS results for 3L therapy are presented in Figure 1. Median OS of non-cell therapy was 7.7 months (CT/CIT: 7.7 months; TT: 7.9 months), and 4.5 months (CT/CIT: 4.5 months; TT: 5.1 months) for 3L and 4L, respectively. Median survival time of pts receiving cell therapy (CAR-T and HSCT) had not reached in either the 3L or 4L setting.
Table 1: Treatment patterns and complete response rates for 3rd Line and 4th Line pts
| 3L | 4L | ||
n (%) | CR rate | n (%) | CR rate | |
CT/CIT | 117 (55) | 9.4% | 66 (52) | 7.6% |
TT | 57 (27) | 8.8% | 44 (34) | 15.9% |
CAR-T | 20 (9) | 60% | 12 (9) | 50% |
HSCT | 18 (8) | N/A | 6 (5) | N/A |

Conclusion
Results from this real-world study illustrate that majority of pts treated with 3L CT/CIT or TT, had poor clinical outcomes, which were exacerbated in the 4L setting. Pts who received cell therapy had better clinical outcomes, however, the proportion of pts receiving these treatments is low. Preliminary data suggests that survival outcomes among CAR-T and HSCT pts, are similar in the 3L and 4L settings. Despite various novel treatments, prognosis for pts with R/R DLBCL remains very poor, underscoring the need for more efficacious treatment options.
Keyword(s): DLBCL, Relapsed lymphoma, Survival, Treatment


